Chapter: Aquaculture Engineering : Ammonia Removal
Chemical removal of ammonia: Principle, Construction - Aquaculture Engineering
Chemical removal of ammonia
Principle
Ion exchangers have sometimes been used in aquaculture to remove ammonia. Ion exchangers utilize the fact that different ions have different electrical charges. Some substances have the ability to attract specific ions in the water because of their charge and exchange them with ions attached less strongly. Ion exchangers may be divided into cation and anion exchangers. For example, cation exchangers are used to remove positively charged calcium and magnesium ions from hard water, while anion exchangers can be used to remove negatively charged nitrate ions. With ammonia, it is the ammonium ion (NH4+) that is removed from the water, so a cation exchanger is required. Removal of ammonium ions ensures a reduction of ammonia levels because of the equilibrium between ammonia and ammonium.
Construction
An ion exchanger comprises a column filled with gravel-like ion exchange substance (Fig. 9.7). The water to be purified, for example by removal of NH4+, flows through this column. The choice of substance to be loaded into the column depends on the ions to be exchanged. To remove NH4+ a clay mineral (zeolite) called clinoptilolite if often used. This mineral occurs naturally in the ground in certain places. The ion exchange substance is deli-vered as a granulate, normally in sizes up to 5 mm. When using clinoptilolite, Na+ is used as the exchange ion and NH4+ is attracted to the ion exchange substance (R−). The following equation then applies:
NH4++ R− Na+⇔ Na++ R− NH4+
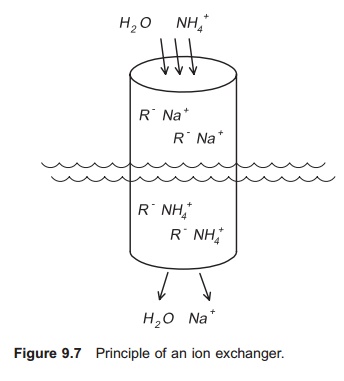
When water with high ammonia content enters the ion exchanger, NH4+ will be attracted to the ion exchange substance and Na+ will be released. After a period of time all the Na+ in the ion exchange sub-stance will have been released and the exchange process will stop; all the NH4+ in the water will flow directly through the column, and the outlet concentration will be the same as the inlet concentration. The ion exchanger will then have to be regenerated by removing all the NH4+ bound to the ion exchange substance. This can be carried out by allowing a regenerating solution containing a very high concentration of sodium ions, for example a salt solution, to flow through the ion exchanger (Fig. 9.8). Often the regenerating solution is re-used; in this case a cleaning circuit for the salt solution is necessary. A stripping process may be used to clean the regenerating solution for NH4+.
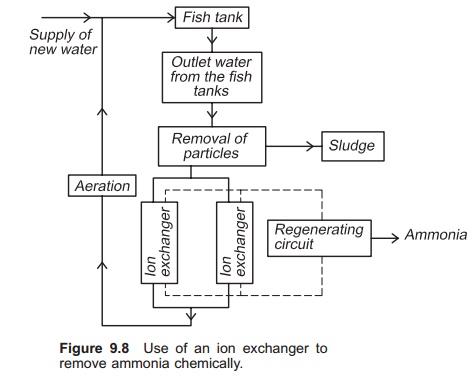
To function optimally the water entering the exchanger must be free of particles. A fine particle filter must therefore be installed before the exchanger. Furthermore, the substance in the ion exchanger must be disinfected at specific intervals to avoid the exchanger starting to function as a biological filter. This is because biofilm will be established on the surface of the exchange sub-stance. The capacity as a biofilter will be very low compared to the exchange capacity. Clogging and blockage will also occur rapidly.
Even if the ion exchanger can remove up to 95% of the ammonia from freshwater, the economic yield of the system is normally negative. The system requires several columns with separate regenerating circuits so that maintenance can be carried out, and to avoid breakdown of the whole system if a column is out of order. Because of this, very few fish farms use ion exchanger technology today. The advantage of an ion exchanger com-pared to a biofilter for nitrification, is that there is no start-up time and no waste product such as nitrate. There have been examples where this tech-nology has been used in relation to long distance fish transport.
Related Topics