Chapter: Plant Biology : Growth and development
Biochemistry of plant growth regulation
BIOCHEMISTRY OF GROWTH REGULATION
Key Notes
Hormones in plants
Plant hormones or ‘growth substances’ are compounds that act specifically to regulate growth and development at low concentrations. Each plant hormone regulates a variety of processes; the range of concentrations over which they act is broad and there is frequently no clear separation between point of synthesis and point of action.
Auxins
Auxins have a variety of effects including elongation growth, cell division and differentiation, and apical dominance. They frequently work with other hormones, principally cytokinins. Plants show polar (directional) auxin transport. Non polar transport also occurs in phloem.
Ethylene
Ethylene (ethene) is a gaseous hormone first identified as a regulator of fruit ripening. It also stimulates senescence and abscission. In seedlings, it initiates the triple response: epinasty, lateral growth and inhibition of elongation. It is synthesized from S-adenosyl methionine (SAM) via 1- aminocyclopropane-1-carboxylic acid (ACC).
Gibberellins
Gibberellins are a large group of compounds formed from isoprene units. Gibberellins stimulate elongation in dwarf plants and mediate the transition from rosette form to flowering in response to temperature or daylength. They also modulate processes involved in seed and bud dormancy.
Cytokinins
Cytokinins promote cell differentiation and division, often acting in association with auxin. They delay senescence, and promote chloroplast maturation in etiolated seedlings. Their synthesis, based on isoprene units, occurs in various tissues and organs. They are transported in the xylem as cytokinin conjugates and inactivated by oxidation.
Abscisic acid
Abscisic acid is a regulator of dormancy and germination of seeds and of plant responses to stress. It is synthesized in roots and shoots, at high rates in stressed tissue. It is transported in xylem and phloem.
Polyamines
Polyamines, like putrescine and spermidine, are compounds with more than one amine group, synthesized from lysine and arginine. They have effects in cell growth and development and in stress responses.
Brassinosteroids
Brassinosteroids are derived from the sterol campesterol. They are present in plants at low levels but stimulate cell division and elongation.
Oligosaccharides
Oligosaccharides, carbohydrate fragments released from plant cell walls, have been shown to elicit plant defenses against fungal attack and may be regulators of development.
Hormones in plants
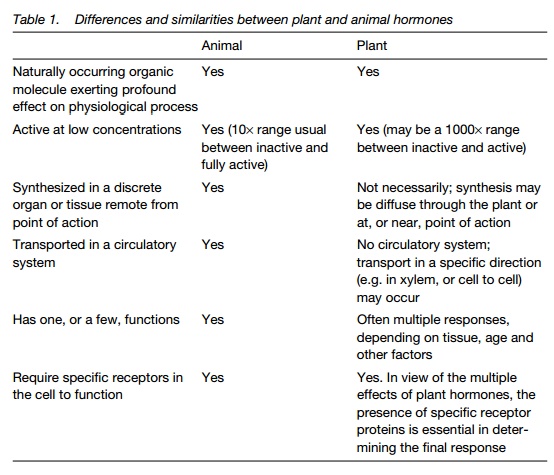
A hormone is defined as a naturally occurring, organic substance that, at low concentration, exerts a profound influence on a physiological process and is not a part of a major metabolic pathway. The plant hormones coordinate processes as diverse as development of the embryo and response to stress. As plant development shows some major differences from animal development (Topic F1), it is not surprising that plant hormones have many differences in mode of action and nature from mammalian hormones. To avoid this confusion, other terms have been used, such as plant growth substance (PGS) and phytohormone. A summary of the key features of plant hormones, and how they differ from animal, is presented in Table 1.
Auxins
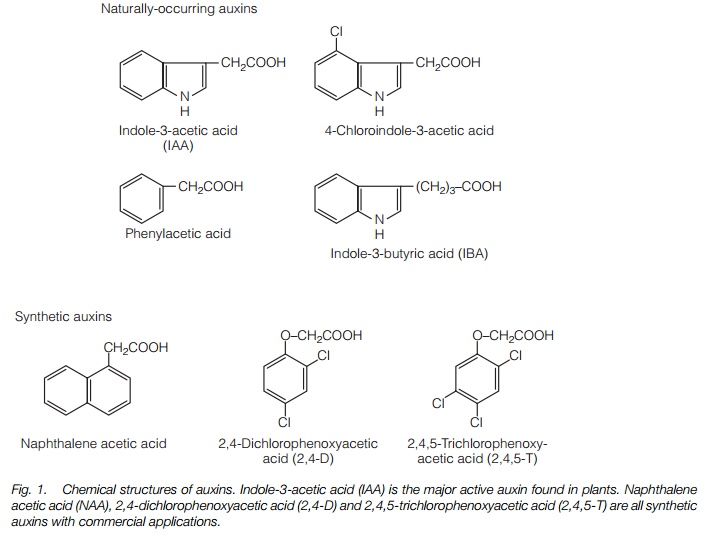
The major plant auxin is indole-3-acetic acid (IAA). A number of other compounds with auxin activity include phenoxyacetic acid and indole 3-butyric acid (Fig. 1).
Auxin effects
Elongation growth. The primary effect of auxin is to regulate stem growth. It does this by stimulating the growth of cells in the direction of elongation. Shoot growth is stimulated by 10–10–7M auxin. Root elongation, on the other hand, is much more sensitive, with maximum stimulation of growth at 10–9–10–10M auxin, and inhibition at higher concentrations.
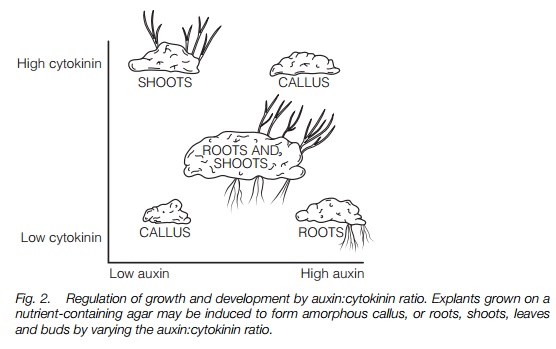
Cell division and differentiation. When callus, an amorphous mass of undifferentiated cells (Topic F1) is grown on an agar plate containing nutrients, the degree of cell division and differentiation to form roots and shoots can be varied by altering the auxin:cytokinin ratio. Both hormones are required; Figure 2 summarizes the results of such an experiment. These effects are also found in plants where auxins induce lateral root formation in stem cuttings.
Apical dominance. A characteristic of the growth of many plants is the dominant growth of the apical bud. When this bud is removed, growth of axillary buds formed a little way behind the apex is stimulated, until one of them becomes dominant and the growth of the others is suppressed. Replacement of the apical bud with auxin inhibits the axillary buds, suggesting that high auxin concentrations generated at the apex inhibit axillary buds. Application of cytokinin to axilliary buds releases them from inhibition and therefore auxin–cytokinin interactions are responsible for the phenomenon.
Other auxin effects. Auxin has a range of other effects, either alone or with other hormones, including fruit development. Some plants, e.g. strawberry, tomato, cucumber, pumpkin, citrus fruits, produce parthenocarpic (seedless) fruits if they are treated with auxin. Senescence and abscission of mature leaves, fruits and flowers is inhibited by auxin; however, abscission of young fruits is enhanced by auxin treatment.
Commercial applications
Synthetic auxins find widespread application in agriculture and horticulture. At high concentrations, 2,4-dichlorophenoxyacetic acid (2,4-D) and2,4,5-trichlorophenoxyacetic acid (2,4,5-T) (Fig. 1) are used as herbicides, particularly on broad-leaved plants, which are much more sensitive to them than monocots. Naphthalene acetic acid (NAA) is used to stimulate rooting of cuttings (‘hormone rooting powder’), while other synthetic auxins are used to reduce fruit number early in the season in apples and to promote fruiting in tomatoes and citrus fruits.
Synthesis
Auxins are mostly synthesized from the amino acid tryptophan, predominantly in young leaves, shoot meristems and developing fruits, wherever cells are dividing rapidly. IAA is also made by bacteria (see Agrobacterium tumifaciens,
Topic P3) and several pathways exist, including one in which IAA is synthesized from indole or indole-3-glycerol phosphate rather than tryptophan.
Auxin transport
Auxin shows polar transport (unidirectional). It moves basipetally (from the apex to the base) in isolated coleoptiles (the sheath encasing the primary leaf in a grass) and stems. Small amounts of auxins produced at the root apex may also move basipetally (in this case from the root tip up the root) but this is limited in comparison with that from the shoot. Polar transport in stems occurs in the parenchyma surrounding the vascular tissue involving specific auxin transportproteins. Its transport can be inhibited by auxin transport inhibitors such as 1-N-naphthylphthalamic acid(NPA). Auxin synthesized in the leaves is also transported in a non-polar fashion in the phloem; this process is about 10 times faster than polar transport. Studies in Arabidopsis have revealed a gene, AUX1,expressed in the root apex, which encodes an auxin transport protein which is involved in directional elongation, for instance in gravitropism (Topic G2).
Auxin conjugation and degradation
The amount of auxin available in a cell or tissue depends on three processes. The rate of auxin biosynthesis or import from other cells, the rate of auxin degradation and the amount that is conjugated (chemically bound) to other molecules. Conjugated auxin is not biologically active. Most auxin within a plant is covalently bonded to organic compounds (e.g. esters of myo-inositol and glucose, and high-molecular weight compounds such as glycoproteins) and is inactive. Transport of IAA in the phloem is predominantly in the form of these complexes, and their breakdown to release IAA supplies it to tissues like the coleoptile tip. There are several pathways for IAA breakdown, involving peroxidation of IAA to 3-methyleneoxindole and non-decarboxylation to oxindole-3-acetic acid.
Ethylene
Ethylene (ethene) was discovered in the early 1900s as a gas that regulated fruit ripening. It had been realized that the close proximity of ripe fruit, such asoranges or apples, speeded up the ripening of other fruits, such as tomatoes andbananas. Regulating ripening, and therefore ethylene, has become an importantpart of the storage, transport and marketing of fruit worldwide. Ethylene has avariety of other roles in plants, including senescence of leaves and fruit, elongationof roots, and responses to waterlogging and other stresses. Although asimple molecule, its effects are highly specific.
Ethylene effects
Fruit ripening. Many ripening fruits show a rise in ethylene production that precedes the onset of ripening. Fruits that produce and respond to ethylene in ripening are the climacteric fruits (apples, tomatoes and bananas); the climacteric is a characteristic burst of respiration that occurs just before the final stages of ripening take place. Ethylene production in climacteric fruit is autocatalytic, i.e. ethylene stimulates its own production, the rapidly rising ethylene concentration then triggering the rapid burst of respiration.
The triple response. Ethylene-treated shoots (e.g. pea seedlings) show three characteristic growth responses simultaneously: epinasty (downward curvature of the leaves); decreased elongation and lateral cell expansion (i.e. increase in stem width) and loss of gravity response to give horizontal growth. Ethyleneinduced epinasty gives the apex of young dicot seedlings a ‘hook’ like appearance.
Ethylene and waterlogging responses. Whereas ethylene normally inhibits elongation growth, in some wet-land species, including rice, ethylene induces rapid elongation growth, allowing the plant to reach air. The formation of
aerenchyma (air spaces in the root cortex, formed by programmed cell death; Topic C1) is also induced by ethylene, which is synthesized in response to low oxygen and accumulates in waterlogged roots.
Other roles of ethylene. High concentrations of ethylene (>10 μl l–1) induce adventitious rooting and root hair formation. In leaf abscission, ethylene accelerates the synthesis of cell-wall degrading enzymes in the abscission layer, a specialized layer of cells at the leaf pulvinus which separate from adjacent cells permitting the leaf to fall from the plant.
Synthesis and degradation
Ethylene is produced from the amino acid methionine, via S-adenosyl methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC). The enzymeproducing ACC is key in regulating ethylene production; it has a very short halflifeand the expression of its gene is stimulated by factors known to induceethylene responses. Ethylene is inactivated by oxidation (e.g. to ethylene oxide orCO2) or it can diffuse from the plant. Rates of ethylene production rise rapidly intissues subject to stress or wounding and subsequently decline to normal levels.
Ethylene is active at very low concentrations (around 1 ppm or 1 μl l–1).
Gibberellins
Gibberellins were discovered in the 1930s by Japanese scientists investigating a disease of rice caused by the fungus Gibberella fujikuroi, that results in tall, seedless plants. Nearly 100 gibberellins have been identified in plants though many do not have biological activity. The most studied, and probably most significant gibberellin is GA3. In common with the other gibberellins, it has a structure based on ent-gibberellane (Fig. 3).
Gibberellin effects
Environmental responses. Many species remain as rosette plants until they have been exposed to either low temperatures (vernalization) or a number of long days. Spinach, for instance, retains a short, squat form until day-length increase, when it begins to grow upwards and flower. Gibberellin levels are low in rosette plants, but increase dramatically in response to the changed environment and initiate the growth response. GA1 is most significant in elongation responses and GA9 in flowering.
Seed germination
In some seeds which show dormancy, gibberellin application will break dormancy. In other seeds, gibberellins are essential in coordinating the processes of germination, increasing in activity upon rehydration of the seed
and initiating the activity of the hydrolases which mobilize seed storage reserves. The role of gibberellins in germinating barley is of economic importance in the malting process, part of beer brewing.
Other effects of gibberellins
Gibberellins are involved in regulating the transition from juvenile to mature growth form in some perennial species such as ivy (Hedera helix); ininitiating flowering and promoting fruit formation.
Synthesis
Gibberellins are diterpene acids synthesized by the terpenoid pathway. Terpenoids are compounds built of repeating isoprene units:
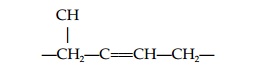
The location of the early stages of gibberellin synthesis is the plastid, where isoprene synthesis occurs from glyceraldehyde-3-phosphate and pyruvate. Later stages occur in plastids in meristems and in enzymes of the endoplasmic reticulum (ER) and cytoplasm.
Transport
Highest levels of active gibberellins in plants are found in young rapidly growing tissues like young leaves and buds, and developing seeds and fruits. Transport of gibberellins in the plant occurs predominantly in the phloem and is non-polar.
Cytokinins
Cytokinins were described as compounds that regulate cell division in plants (Topic B6) in experiments in which compounds were screened for their effects on tissue cultures. From these experiments came two compounds: the first, kinetin, was isolated as the active ingredient in herring sperm DNA that caused massive cell proliferation in plant cell culture. Zeatin was the first natural plant cytokinin, isolated from the liquid endosperm of the coconut. The cytokinins constitute a small number of compounds, which are derivatives of adenine or amino purine.
Cytokinin effects
Cytokinin effects are generally associated with promotion of growth and development and delay of senescence. Cytokinins applied to leaves will delay senescence; they speed up chloroplast maturation in etiolated (dark-grown)
tissue and promote cell expansion in young leaves. Cytokinins applied to lateral buds in plants showing strong apical dominance will overcome growth inhibition by auxin, causing the bud to grow out. Cytokinins, with auxins, are involved in plant tumor formation and in morphogenesis, the development of roots and shoots. Figure 2 illustrates the effects of varying the ratio of auxin to cytokinin on the growth and morphogenesis of plant material in tissue culture.
Cytokinin synthesis
Like gibberellins, cytokinins contain isoprene subunits. The first stage involves the reaction of isopentenyl pyrophosphate with adenosine monophosphate (AMP), catalyzed by the enzyme cytokinin synthase to yield isopentenyl adenine ribotide. From this compound, cytokinin ribotides, ribosides and cytokinins are formed. Cytokinins are also synthesized by gene products resulting from the insertion of bacterial genes fromAgrobacterium tumifaciens (Topic P3) The TI (tumor- inducing) plasmid from A. tumifaciens introduces the gene for isopentenyltransferase. This enzyme generates isopentenyl adenine, which is converted to trans-zeatin and dihydrozeatin in the plant. These hormones, together with auxin produced by another TI-plasmid gene product, cause a tumor or crown gall to form at the site of infection.
Cytokinin transport
Cytokinins are synthesized in various tissues and organs, though the root apical meristem (Topic C2) is a major site of its production. They have been identified in the xylem flow from cut roots, suggesting that this may be a route for longdistance transport of cytokinins through the plant. Cytokinins in the root xylem are predominantly zeatin ribosides, which are rapidly converted to freecytokinin in leaves. Cytokinin inactivation occurs when they are oxidized to adenine by the enzyme cytokinin oxidase.
Abscisic acid
Abscisic acid (ABA) is found in all higher plants and mosses. ABA regulatesdormancy and is central to plant responses to stress. The nature of the moleculemeans that it can exist in several forms. First, the carboxyl group at the end of the side chain may be cis or trans (Fig. 4), while the C at position one of the ring is asymmetric and gives either (+) or (-) (S or R) enantiomers. The active formof ABA is (+) cis ABA; (+)-2-trans-ABA also exists in plants and is active in some long-term ABA responses; it may be converted to the (+) cis form in tissues.
Abscisic acid effects
ABA has a variety of effects related (i) to seed dormancy and (ii) to stress responses. ABA levels rise initially during embryo development within theseed and then decline. ABA regulates the expression of genes for proteins in
the embryo that prepare it for the final stages of seed development in which the seed desiccates and becomes dormant; it also activates genes for seed storage proteins (Topic H4). ABA also keeps some seeds dormant until the environment becomes suitable for growth. Controlling dormancy is very important in temperate climates since precocious germination may lead to the death of the seedling. ABA also accumulates in the dormant buds of woody species, although control of dormancy here is likely to be the result of the action of several hormones.
ABA also regulates several plant stress responses. Rising ABA levels in water stress initially cause stomatal closure (Topic I2) and subsequently increases the ability of root tissue to carry water; it also promotes root growth and inhibits shoot growth.
Abscisic acid synthesis
ABA biosynthesis begins in chloroplasts and amyloplasts. Synthesis, like that of gibberellins and cytokinins, involves the isoprene subunit in isopentenyl pyrophosphate, which is used to produce an oxygenated carotenoid compound,
zeaxanthin. Zeaxanthin is modified in a multi-stage process to neoxanthin, which is cleaved to the C15 compound xanthoxin; xanthoxin is then modified in two stages to produce ABA. ABA is degraded, either by oxidation or by conjugation, to form ABA-glucosyl ester.
Abscisic acid transport
ABA is synthesized in roots and shoots, and at much higher levels in tissues undergoing stress. Water-stressed roots, for instance, produce up to 1000 times more ABA that is transported through the xylem to the shoot. ABA is also transported from shoot to root in the phloem.
Polyamines
Polyamines are compounds containing two or more amine groups. Typical examples with biological activity are:
●Putrescine: H2N-(CH2)4-NH2
●Spermidine: H2N-(CH2)3-NH-(CH2)3-NH2
●Spermine: H2N-(CH2)3-NH-(CH2)3- NH-(CH2)3-NH2
Biosynthesis originates from the amino acids lysine and arginine. Levels of polyamines increase where rapid cell division is occurring; putrescine levels increase in response to some forms of stress and they may be involved in some aspects of embryo and fruit development.
Brassinosteroids
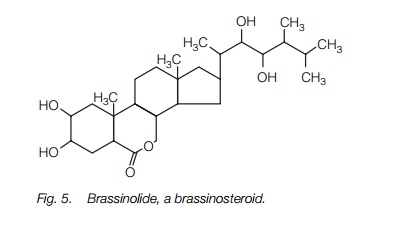
Brassinosteroids (or brassins) are a recently discovered, complex group of lipids synthesized from the sterol campesterol. They are present at low levels, but have strong growth promoting effects, stimulating both cell division and cell elongation. The structure of one brassinosteroid is shown inFig. 5. It appears that brassinosteroids act with the other plant hormones to regulate growth and differentiation. Mutants of Arabidopsis and pea which are deficient in brassinosteroid biosynthesis are dwarf; application of brassinosteroid restores them to a normal phenotype, indicating that they are essential for cell elongation in normal plants.
Oligosaccharides
The complex polysaccharide cell wall of plants is a dynamic structure, which may be modified by both endogenous and exogenous enzymes. Wall changes lead to the release of fragments of long-chain polysaccharides, oligosaccharides, into the apoplast, some of which have been shown to have effects on development in plant tissue cultures and others of which are released during fungalpathogen attacks and elicit the defense responses of the plant (Topics G5 and M4).
Related Topics