Chapter: Clinical Cases in Anesthesia : Open-Eye Injury And Intraocular Pressure
What is the mechanism by which intraocular pressure (IOP) is normally maintained?
What is the mechanism by which intraocular pressure (IOP) is
normally maintained?
IOP contributes to the refracting properties of
the eye.
Significant intraocular hypotension or
hypertension may lead to blurred vision or refractive discrepancies. The normal
range of IOP is 10–22 mmHg. IOP is much higher than tissue pressure, which is
2–3 mmHg, and intracranial pressure, which is 7–8 mmHg. Pressures greater than
25 mmHg are considered abnormal. There are diurnal variations of modest
proportion, 2–3 mmHg. IOP is high-est in the morning secondary to the
dilatation of pupils during sleep, carbon dioxide (CO2) retention,
the recum-bent position, immobility of the eye, and pressure of the eyelid.
Each systole can increase the pressure by another 1–2 mmHg, while inspiration
can lower IOP by 5 mmHg.
IOP is normally maintained by several factors.
One fac-tor is external pressure exerted by periorbital structures, such as the
extraocular muscles, venous congestion of the orbital veins, closure of the
eyelid, or contraction of the orbicularis ocularis muscle. A second factor is
scleral rigidity. The sclera is normally distensible, but as IOP rises it
becomes rigid, thereby exacerbating intraocular hyper-tension. A third factor
is the volume of intraocular fluid, such as blood, aqueous humor, and the
semisolid struc-tures, which include the lens, the vitreous, and intraocular
tumors.
Alterations in fluid contents are crucial to
IOP. Intraocular blood is mainly present in the choroid plexus, and the state
of dilatation or contracture of these vessels will determine the blood volume
in the eye. Although IOP is maintained at a relatively uniform level regardless
of the degree of hypertension, an acute rise in arterial blood pres-sure may
increase IOP. Over time, if arterial hypertension is chronic, IOP will normalize
after adaptation of the choroidal vessels.
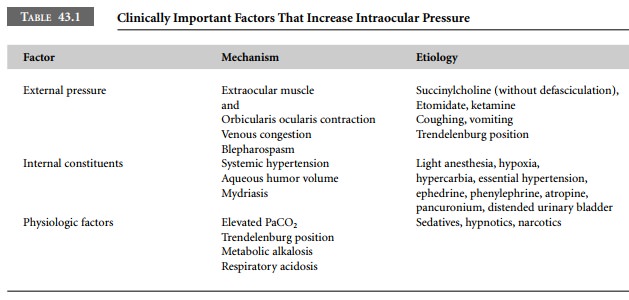
Impaired venous drainage increases IOP.
Coughing or straining will raise IOP by elevating venous pressure (Table 43.1).
A mild cough can raise IOP by 34–40 mmHg. Similarly, retching, coughing, or
vomiting during induction of anesthesia may cause a rise in IOP which persists
for many minutes. This may be particularly true in patients with a history of
smoking.
Another important intraocular constituent is
aque-ous humor, two-thirds of which is formed in the poste-rior chamber, and
one-third of which is produced in the anterior chamber. It is secreted from
epithelial cells of the ciliary process. After formation in the posterior
seg-ment, aqueous humor circulates through the pupil into the anterior chamber,
which is subsequently drained at the angle of the eye and at the spaces of
Fontana. The spaces of Fontana are channels in the trabecular mesh. Impaired
drainage through this trabecular mesh results in glaucoma. Aqueous humor
continues to pass into Schlemm’s canal and the ophthalmic, cavernous, and
jugu-lar veins.
Aqueous humor is similar in composition to
plasma, without its proteins. Aqueous humor secretion is an energy-requiring
process mediated through a sodium pump mechanism. Its production requires both
cytochrome oxidase and carbonic anhydrase. Changes in solute con-centration of
plasma can affect the formation of aqueous humor and, consequently, the IOP.
Thus, mannitol or glycerol is used for lowering IOP. Acetazolamide may also
lower IOP. Each of the aforementioned agents has meta-bolic consequences.
Pupillary dilatation narrows the trabecular
mesh and spaces of Fontana, predisposing the patient to glau-coma. Agents
causing pupillary constriction (miosis) improve aqueous drainage, whereas agents
producing pupillary dilatation (mydriasis) impair aqueous drainage.
Related Topics