Chapter: Clinical Cases in Anesthesia : Transfusion Reaction
What are the immediate and delayed adverse effects of blood transfusion?
What are the immediate and delayed adverse effects of blood
transfusion?
Immediate Reactions
Immediate transfusion reactions are those that
occur during or within 24 hours after transfusion whereas delayed reactions
occur several days to years after the trans-fusion. The immediate adverse
effects of blood transfusion are summarized in Table 49.1.
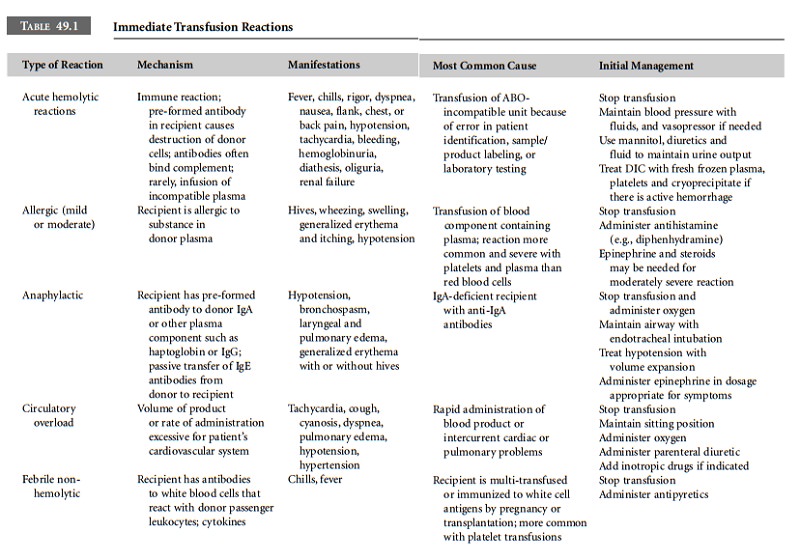
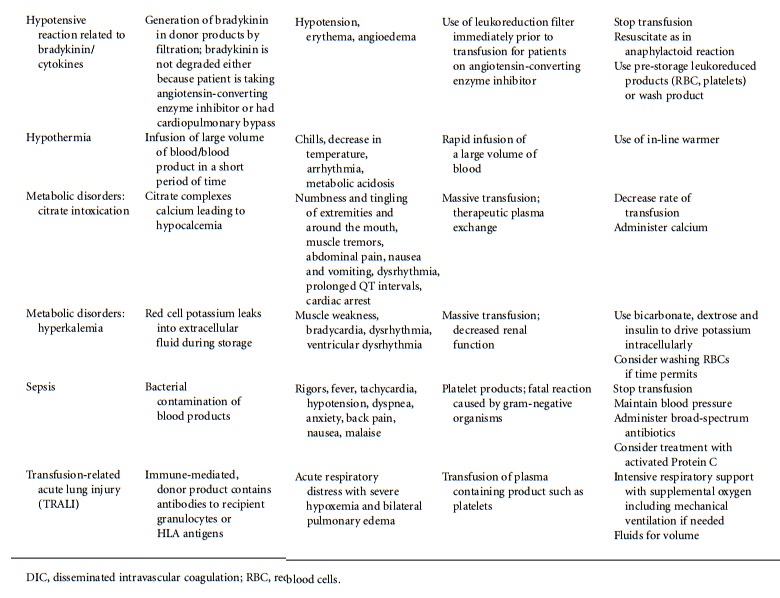
Acute Hemolytic Reaction Acute hemolytic transfusion reactions are the most
frequent cause of fatal transfusion reactions reported to the FDA. These
reactions result from either failure to detect blood group incompatibility or
inadvertent transfusion of a blood product to the wrong patient. The risk of
death from acute hemolytic reactions has been estimated to be
1:587,000–1:630,000 using data reported to the FDA. Data from the French
Haemovigilance system reported approximately 1 death per 2 million units
transfused. Most acute hemolytic reactions are caused by transfusion errors.
Data from the United Kingdom Serious Hazards of Transfusion (SHOT) initiative
indicated that 52% of events investigated involved incorrect transfusion of
blood or blood compo-nent. An analysis of transfusion errors in New York State
that resulted in transfusion of a blood product to other than the intended
recipient indicated that erroneous transfusion occurred in 1 of 19,000 red cell
units trans-fused. The frequency of fatal reactions was 1:1,800,000.
Approximately half the errors occurred at the bedside and involved
administration to the wrong recipient (38%) or phlebotomy errors (13%). Blood
bank errors, including testing the wrong sample, transcription errors, and
issuing the wrong unit, accounted for 29% of errors. Fifteen percent of events
involved multiple errors.
The severity of the reaction is in general
related to the amount of blood transfused. Mortality appears to be directly
related to the volume of incompatible cells transfused. A review of 41 cases of
hemolytic transfusion reactions causing renal failure demonstrated 25%
mortality when 500–1,000 mL of blood was transfused and 44% mortality when more
than 1000 mL was transfused. However, it is important to remember that
fatalities have occurred when as little as 30 mL was transfused. Transfusion
guidelines recommend that patients be monitored carefully during the first
15–30 minutes of a transfusion, so that there will be prompt recognition of a
serious reaction.
It is important that clinicians recognize that
blood group incompatibility, while common, is not the only possible cause of
what appears to be a hemolytic transfu-sion reaction. The patient may have an
intercurrent illness causing in vivo destruction of red cells. Autoimmune
hemolytic anemias, congenital hemolytic anemias, drug-induced hemolysis,
microangiopathic hemolytic anemias, and infections such as malaria or
babesiosis where red cells are parasitized or clostridial infections where
toxins hemolyze red cells, are among the disorders that may mimic an acute
hemolytic transfusion reaction. Ventricular assist devices, membrane
oxygenators, and artificial heart valves may also cause hemolysis. Factors that
could cause non-immune hemolysis may also need to be considered. Improper
storage of blood (very low temperatures at which blood freezes) or malfunction
of a blood warmer may cause thermal destruction of red cells before
transfusion. Infusing blood under pressure or through a small-gauge needle may
also cause hemolysis. Finally, incompatible intravenous solutions will cause
hemolysis if administered with blood.
Allergic and Anaphylactic Reactions Mild allergic reactions manifested
primarily by urticaria are common following blood transfusion, occurring in
approximately 1–3% of transfusions. Severe allergic reactions associated with
hypotension, respiratory distress, and other cardiac and gastrointestinal
symptoms of anaphylaxis are esti-mated to occur in 1:20,000 to 1:47,000
transfusions. In the French Haemovigilance system approximately 31% of reported
events were allergic in nature. In most allergic reactions, patients appear to
have preformed antibodies to a component of donor plasma, usually a plasma
protein. In severe reactions, the patient may be IgA-deficient and have
anti-IgA antibodies. On occasion the patient will have IgA but will lack one of
the isotypic or allotypic determinants of the IgA class. Other patients have
antibodies to other immunoglobulin classes or to proteins such as haptoglo-bin.
Passive transfer of allergens or passive transfer of IgE antibodies in the
donor product should also be considered. Although allergic reactions are
generally thought to be IgE-mediated and histamine release from mast cells is
the primary mediator, IgE antibody is not always demonstra-ble.
Complement-derived anaphylatoxins such as C3a and C5a generated by immune
complexes or other secondary mediators such as cytokines are responsible for
anaphylac-toid symptoms.
Mild allergic reactions are usually treated
with an oral or parenteral (intravenous or intramuscular) antihista-mine
(diphenhydramine 25–50 mg). If a patient has a documented history of recurrent
allergic reactions, prophy-lactic administration of an antihistamine is
indicated. The mild allergic reaction may be the only reaction where there is
general agreement that the transfusion could be inter-rupted, an antihistamine
given, and the transfusion restarted when symptoms subside. Severe allergic or
ana-phylactoid transfusion reactions should be treated as any other type of
anaphylaxis is treated (i.e., epinephrine, volume expansion, oxygen
supplementation and other respiratory support, etc.). Any patient having an
anaphy-lactoid reaction should be tested for antibodies to IgA. Patients who
have IgA antibodies must receive plasma and platelets from IgA-deficient
donors. These products are usually available only from large regional blood
centers. Red blood cells washed with 2 liters of normal saline will be
satisfactory for transfusion. IgA-deficient patients who may need plasma
derivatives pose a special problem because the standard labeling on these
products does not indicate whether there may be trace amounts of IgA pres-ent.
As little as 1 mg of IgA may trigger an allergic reaction. Clinicians should
speak with the manufacturer’s represen-tative to determine whether a particular
product and lot number is safe.
Circulatory Overload Circulatory overload is a frequent complication
of transfusion therapy. It usually occurs in elderly patients with renal
insufficiency, diminished car-diac reserve, or severe anemia. Circulatory
overload is often included in acute pulmonary problems in many reports and
accurate data on the incidence are not available. However, a report from the
Mayo Clinic suggested that it occurred in 1/3,168 patients receiving red cell
transfusions. The early symptoms of volume overload are nonspecific and include
an increase in blood pressure, headache, devel-opment of a new cough, and a
sensation of pressure in the chest. The development of any of these signs and
symp-toms in a patient receiving transfusion therapy should be an indication to
slow the infusion and monitor the patient more often. As cardiac decompensation
progresses, dyspnea, orthopnea, tachycardia, cyanosis, and frank pulmonary
edema may develop.
When the patient’s signs and symptoms suggest
circula-tory overload, the transfusion should be stopped and the patient placed
in a sitting position. Oxygen supplementa-tion should be provided and diuretics
given to reduce the intravascular volume. If diuretics are ineffective,
consider-ation should be given to phlebotomy.
Febrile Non-hemolytic Reactions The definition of a febrile non-hemolytic
reaction is an increase in tempera-ture of ≥1°C following transfusion that cannot be
explained by the patient’s clinical condition. This increase in temperature is
usually accompanied by chills and rigors and sometimes headache, nausea, and
vomiting. In most patients, fever develops during the transfusion. Usually the
increase in temperature is ≤2°C. When the temperature increase is ≥2°C, bacterial contamination of the blood product and the development
of an intercurrent infection must be considered. Some patients have chills,
rigor, and feel cold but do not develop fever. The diagnosis of a febrile
non-hemolytic reaction can be established only by exclud-ing other types of
transfusion reactions accompanied by fever. In a community hospital population,
approximately 0.5–1.0% of red cell transfusions are associated with febrile
non-hemolytic reactions. In chronically transfused patients, the frequency is
much higher.
Recipient antibodies directed against antigens
on donor leukocytes are generally considered to be the cause of febrile
non-hemolytic reactions. Initially it was believed that endogenous pyrogens
(interleukin-1β, interleukin-6, and tumor necrosis factor) from donor leukocytes
caused the febrile reaction. Recently, it has been suggested that complement
activation following the interaction of recipi-ent antibodies with donor
leukocytes causes activation of recipient monocytes. These activated monocytes
are thought to release proinflammatory cytokines causing the reaction. Finally,
since the widespread use of leukoreduc-tion filters to eliminate these
reactions, it appears that cytokines produced by donor leukocytes prior to
leuko-reduction may also cause febrile non-hemolytic reactions.
In the general population, only 15% of patients
having a febrile reaction to a red cell product are likely to have a recurrent
febrile reaction with the next transfusion. Therefore, many transfusion
services do not recommend premedication or leukoreduction for the average
patient until a second febrile reaction occurs. Febrile reactions are more
common following platelet transfusions than red cell transfusions and are more
common with older products than relatively fresh products. If reactions are
mild, they can often be prevented by premedication with an anti-pyretic. If
reactions are severe or if premedication does not prevent the reaction,
leukoreduced products are indicated. In some multitransfused patients, it may
be necessary to provide pre-storage leukoreduced products. Pre-storage
leukoreduction will not remove all biologically active mediators because some
of these are derived from platelets. Removal of most of the plasma from
platelet products just prior to transfusion helps reduce reactions in patients
not responding to pre-storage leukoreduction strategies.
Other Hypotensive Reactions Hypotension not uncom-monly accompanies severe
immune reactions in which antibodies in the recipient react with donor cells or
vice versa and transfusions where bacterial contamination of a blood product
has been documented. In addition to these circumstances, hypotension has been
described following the administration of leukoreduced products (both platelets
and red cells), following the administra-tion of plasma-containing products to
patients taking angiotensin-converting enzyme (ACE) inhibitors and fol-lowing
the administration of plasma protein fraction to patients undergoing
cardiopulmonary bypass. These reac-tions appear to be related to the generation
of bradykinin under circumstances where enzymes important in bradykinin
inactivation are either inhibited or a tissue con-taining these enzymes has
been excluded from the circula-tion (cardiopulmonary bypass). Contact of plasma
with negatively charged blood filters leads to contact activation of the
intrinsic coagulation system and bradykinin genera-tion. There are at least
five metallopeptidases responsible for the inactivation and metabolism of
bradykinin. ACE is responsible for the hydrolysis of 60% of bradykinin in
normal subjects and inactivation by aminopeptidase P (APP) is a second major metabolic
pathway. Clinical reports have documented unexplained hypotensive reac-tions in
patients on ACE inhibitors receiving platelet prod-ucts leukoreduced at the
bedside and in patients under-going therapeutic plasma exchange. In vitro
studies have documented an increase in bradykinin levels following fil-tration
of platelet concentrates. The generated bradykinin was rapidly degraded and was
undetectable after 1 hour of storage.
Treatment of a hypotensive reaction in a
patient on ACE inhibitors should include immediate discontinuation of the
product and appropriate resuscitation. If additional red cells are needed,
washing is advised. If platelets are needed, the product should be pre-storage
leukoreduced or filtered an hour prior to administration. Reduction of the
residual plasma on the platelet product may also be helpful.
Transfusion-Transmitted Bacterial Infection Sepsis related to bacterial contamination
of blood products is the second most common cause of fatal transfusion
reactions reported to the FDA. In the United States between 1976 and 1999, 10%
of transfusion-related deaths were caused by bacterial contamination. It is
estimated that 0.2% of whole blood collections are contaminated. Bacterial
contamination of platelets is more common than contam-ination of red blood
cells but prevalence estimates vary widely. Estimates for red cells vary from
0.002% to 1.0% and for platelets from 0.04% to 10%. Some studies have suggested
that contamination of pooled platelet concen-trates is more frequent than contamination
of single donor platelets. The organisms contaminating blood products are
usually from donor skin flora (i.e., Staphylococcus
species, Propionibacterium acnes).
However, asymptomatic donor bacteremia
(Yersinia enterocolitica) and
contamination of products from environmental sources (Pseudomonas species) may also lead to bacterial contamination of
blood components.
The most common symptoms and signs associated
with bacterial contamination of blood products are chills, fever, tachycardia,
shock or hypotension, shortness of breath, back pain, and nausea and/or
vomiting. Occasional patients may have an increase in blood pressure. Although
these symptoms often develop immediately or within the first hour after
transfusion is initiated, some patients may not develop symptoms or signs for
several hours.
Two national studies of bacterial contamination
of blood products performed in France and the United States provide the most
current data on this complication of transfusion. In the United States from 1998
to 2000, suspected cases of bacterial contamination of blood prod-ucts were
reported to the Centers for Disease Control (CDC) by blood collection
facilities and transfusion serv-ices associated with the American Red Cross,
the American Association of Blood Banks, and the Department of Defense. This
study was given the acronym BaCon (Assessment of the Frequency of Bacterial
Contamination Associated with Transfusion Reaction). In France, there is a
national mandatory reporting system for adverse reactions to transfusion. From
November 1996–1998, all adverse reactions suspected to be related to bacterial
contamina-tion were investigated as part of the French BACTHEM Study.
The case definition for BaCon included the
presence of one or more of the following signs or symptoms develop-ing within 4
hours of transfusion: fever ≥39°C or a change of ≥2°C from the pre-transfusion value; rigors;
tachycardia ≥120 beats per minute, or a change of ≥40 beats per minute from the pre-transfusion
value; a rise or drop of ≥30 mmHg in systolic blood pressure. Of 56
reported cases, 34 were confirmed with the same organism being cultured from
the recipient and the component. There were 9 deaths. The rate of
transfusion-transmitted bacteremia (in events per million units distributed)
was 9.98 for single donor platelets, 10.64 for pooled platelets, and 0.21 for
red cells. The rate of fatal reactions was 1.94 for pooled platelets, 2.22 for
single donor platelets, and 0.13 for red blood cells. Fatal transfusion
reactions were more likely to be associ-ated with contamination with
gram-negative organisms.
Results from BACTHEM were similar. Of 158
suspected cases, 41 were confirmed. Twenty-five were associated with red blood
cells and 16 with platelet products. This led to an estimated incidence rate
per million components issued of 5.8 for red blood cells, 31.8 for apheresis
platelets, and 71.8 for pooled platelet concentrate. Fatal reactions were
uni-formly associated with gram-negative organisms.
In comparing results from current studies and
earlier published reports, the organisms identified as contaminants have
changed over time. In earlier studies, Yersinia
enterocol-itica and Pseudomonas species
were the predominant organ-isms isolated from red cells. In the BaCon study Serratia species accounted for most of
the cases of red blood cell contamination and Acinetobacter was the second most frequent species cultured from
blood components in the BACTHEM study. Currently, manufacturers are focusing on
the development of systems to detect bacterial contamination in blood products
and the development of nucleic acid bind-ing compounds that will prevent the
proliferation of bacteria accidentally introduced into units at the time of
phlebotomy.
Transfusion-Related Acute Lung Injury Transfusion-related acute lung injury (TRALI)
is one of the most serious, underdiagnosed complications of transfusion.
Typically, acute respiratory distress develops within 1–2 hours of starting a
transfusion of a blood component that contains plasma, but some patients have
developed symptoms as late as 6 hours after transfusion. Patients have severe
hypoxemia and pulmonary edema. Hypotension and fever may also occur. The SHOT
study from the UK reported that 11 of 169 (7%) reports involved acute lung
injury. Data from the Mayo Clinic suggest that the incidence of TRALI may be
1:5,000 with plasma-containing transfusions. In Canada, data from Quebec
reported 3 cases of TRALI in a population receiving 190,000 red cells and 3,000
units of plasma. At a hospital in Ontario, one case of TRALI was observed
annu-ally and 12,000 red cell units were transfused.
In most investigations, donor plasma has
contained anti-bodies either to granulocytes or to HLA antigens (both class I
and class II). In a small percentage of cases, the recipient serum has
contained such antibodies (prior to transfusion). However, antibodies have not
been identified in all cases, and it has been hypothesized that lipid products
from neutrophils may cause TRALI. A case of TRALI following the administra-tion
of intravenous immune globulin was recently reported. Treatment for TRALI
depends on the severity of the reaction. Supplemental oxygen and respiratory
support including mechanical ventilation may be required. Hypotension is
cor-rected and corticosteroids are usually given.
When a blood product is implicated in TRALI, a
donor serum sample is obtained and tested for antibodies to leukocytes. Most
such donors are multiparous women. When antibodies are identified, donors are
usually no longer allowed to donate. If they have an unusual blood type, the
red cells are collected and either frozen or washed, processes that eliminate
residual plasma.
Delayed Reactions
Delayed adverse reactions to blood transfusion
may be immunologic, related to transmission of a viral or parasitic disease, or
caused by iron overload secondary to trans-fusion. Immunologic adverse effects
include delayed hemolytic transfusion reaction (DHTR), immunization to red
cell, platelet, leukocyte or plasma antigens, auto-immune phenomena triggered
by alloimmunization, and graft-versus-host disease.
Delayed Hemolytic Transfusion Reactions Delayed hemolytic transfusion reactions
usually result from failure to detect existing antibody because the antibody
concentration has dropped below the detection level for the method used in
antibody screening and crossmatching tests. The trans-fusion stimulates
antibody production causing an acceler-ated destruction of transfused cells.
Common presenting signs and symptoms include unexplained fever, a decrease in
hemoglobin unexplained by clinical events, and an increase in bilirubin several
days to weeks after transfusion. When a patient blood sample is examined, a
positive direct antiglobulin test is present and often antibody can be eluted
from the transfused cells. Antibody may also be detectable in patient serum at
this time. Delayed reactions are usually mild and may go unrecognized.
Clinically detectable hemolysis is reported to vary from 1:5,000 to 1:10,000
transfusions. Some delayed reactions are serious, causing disseminated
intravascular coagulation, renal failure, and death. In the first 2 years of
the SHOT study, 51 of 366 (14%) reports concerned delayed hemolytic transfusion
reactions; and in two cases death was attrib-uted to the transfusion reaction.
Alloimmunization Alloimmunization to antigens on red cells
is estimated to occur in 1–1.6% per donor unit provided that D-negative units
are given to D-negative recipients. However, immunization may become a serious
problem when a patient requires chronic transfusion and the red cell phenotypes
of the blood donors vary from that of the recipient population. For example,
most patients with sickle cell disease are African-American and in most
communities in the United States the majority of blood donors are Caucasian.
Some specialists in the treatment of sickle cell disease have recommended
prospective match-ing of clinically important blood groups for patients on
chronic transfusion protocols to prevent immunization and lower the risk of
delayed transfusion reactions. Immunization to HLA antigens through routine
blood transfusion may also pose problems for patients. Red blood cells and
platelet products contain a large number of white blood cells that normally
express HLA antigens. Patients having many transfusions with unmodified red
cell or platelet products may become immunized to antigens of the HLA system.
Immunization to HLA antigens will make a patient with thrombocytopenia
refractory to random donor platelet transfusions and may make finding a com-patible
solid organ donor impossible for a patient needing a heart, kidney, or small
bowel transplant. The prophylactic use of leukoreduced red cell and platelet
products may be indicated for these patient populations.
Immunization to platelet-specific antigens may
also occur following transfusion. In addition to refractoriness to platelet
transfusions this type of immunization may lead in rare instances to the
disorder post-transfusion purpura. In post-transfusion purpura patients develop
severe throm-bocytopenia 5–10 days following transfusion. Patients are usually
women who have been previously pregnant or transfused. Although several
platelet-specific antigen systems have been reported to cause this disorder, in
most cases patients lack a platelet-specific antigen HPA-1a (PlA1),
and have made an anti-HPA-1a. HPA-1a is a high-frequency platelet-specific
antigen with only 2% of the population being HPA-1a-negative. Through
mechanisms that are not well understood the anti-HPA-1a destroys the transfused
HPA-1a-positive transfused platelets and the patient’s own HPA-1a-negative
platelets. Post-transfusion purpura is usu-ally self-limited with spontaneous
recovery within 3 weeks. Occasional patients with severe symptomatic
thrombo-cytopenia require treatment. High-dose intravenous immune globulin
(IVIG) is the treatment of choice. Random platelets are generally
HPA-1a-positive and will not increase the platelet count. Antigen-negative
platelets may be helpful when given with IVIG.
Graft-Versus-Host Disease Transfusion-associated graft versus-host
disease (TA-GVHD) is a rare but usually fatal complication of transfusion in
which viable lymphocytes in donor blood products transfused to an
immunologically compromised patient engraft. These foreign lymphocytes recognize
the HLA antigens of the transfusion recipient as foreign and generate a
characteristic immune response characterized by rash, fever, liver function
abnormalities, diarrhea, and marrow dysfunction. Symptoms usually develop 8–10
days after transfusion. TA-GVHD was origi-nally described in immunocompromised
individuals. It was described in newborns having exchange transfusion, patients
with congenital forms of immune deficiency, and patients immunosuppressed by
intense chemotherapy. Subsequently, it was reported in immunologically normal
individuals transfused with blood from an individual who was either
HLA-identical or homozygous for a shared HLA haplotype. The risk of such
matched transfusions in unre-lated populations varies.
Treatment of TA-GVHD is rarely effective, with
90% of patients dying from the disorder. Transfusion guidelines are focused on
prevention through blood product irradia-tion. Irradiation of blood products
requires a specific physician order. However, most blood bank/transfusion services
have developed guidelines for blood product irradiation. Transfusions to
premature infants, neonates, individuals with congenital immune deficiency,
recipients of hematopoietic stem cell transplants, patients receiving intense
immunosuppressive chemotherapy (for leukemia, Hodgkin’s disease, and other
lymphomas), and directed blood product donations from family members are
gener-ally irradiated routinely. Physicians should be informed about
irradiation guidelines at the institutions where they practice. Products for
some patients may automatically be irradiated based on admitting diagnosis but
other cases may require a specific order. It is always best to specifically
order irradiated blood, rather than to depend on a stan-dard operating
procedure. This practice minimizes the opportunity for error.
Iron Overload Transfusion hemosiderosis is common when
patients with hematologic disorders require chronic transfusion. Typically
these are patients with hemo-globinopathies such as thalassemia or sickle cell
disease. However, patients with myelodysplastic syndromes are also at risk for
this disorder. Chronically transfused patients should have ferritin levels
monitored and should be treated with iron-chelating agents such as
desferoxamine when needed.
Transfusion-Transmitted Viral and Parasitic
Infection Currently
blood donations for allogeneic use have been tested by FDA-licensed tests and
found negative for antibodies to human immunodeficiency virus (anti-HIV),
hepatitis C virus (anti-HCV), human T-cell lym-photrophic virus (anti-HTLVI/II)
and hepatitis B core antigen (anti-HBc), as well as HIV-antigen (HIV-1-Ag, also
called p24 antigen) and hepatitis B surface antigen (HbsAg). Beginning in March
1999, nucleic acid amplifica-tion testing (NAT) for HIV and HCV has been
performed on most of the blood collected in the United States under an
investigational new drug application (IND) approved by the FDA. Traditional
tests are performed on individual samples, but NAT testing is performed on
pooled samples. Two manufacturers have developed test systems using NAT.
Gen-Probe (San Diego, CA) developed a multiplex system that tests for HCV and
HIV at the same time. Roche Molecular Systems (RMS, Pleasanton, CA) developed
inde-pendent NAT tests for HIV and HCV. Since the imple-mentation of NAT under
FDA IND one case of HIV transmitted by blood transfusion has been reported in
the United States. Although the new tests are very sensitive, the window period
for HIV remains at 10–11 days. NAT testing is not yet legally mandated, but
will be as soon as suf-ficient licensed kits are available to screen the entire
US blood supply. In the meantime, the FDA is requiring that all allogeneic
units be NAT-tested unless there is an extremely urgent situation such as the
9/11 terrorist attacks. At the present time, NAT testing for HBV on pooled
samples does not appear to be superior to serologic tests for HbsAg. It is
unclear when NAT testing for HBV will be introduced.
The Gen-Probe test for HIV and HCV was licensed
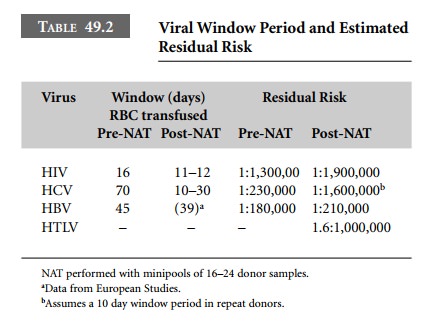
in February 2002. Licensure of the RMS test is imminent. Table 49.2, derived
primarily from the American Red Cross experience, provides data on the window
period reduction with NAT using pooled testing for transfusion-transmitted
viruses. For HIV the residual risk with NAT decreased from 1:1,300,000 to
1:1,900,000 per million red cells transfused. For HCV, the window period could
become 10–12 days. Although the estimated reduction in the window period for
HCV (time from infection to seropositivity) is calcu-lated to be 32.5–40 days,
there is a very high level of viremia in HCV infection, with virus becoming
detectable 10–12 days after exposure. Thus NAT may reduce the HCV window period
to 10–12 days. If these estimates are correct, the residual risk could be as
low as 1:1,600,000.
Blood is not routinely screened for
cytomegalovirus (CMV) or parvovirus B19 even though these infections can be
transmitted by transfusion. However, the standard of care is to provide
CMV-seronegative blood for individuals who are CMV-seronegative and are either
immunodefi-cient (infants, congenital or acquired immunodeficiency) or are
candidates for hematopoietic stem cell transplanta-tion or solid organ
transplantation where the tissue donor is also CMV-seronegative. Blood centers
screen part of their inventory for CMV to provide CMV-seronegative blood for
these special patients. Most adult blood donors are CMV-seropositive. The
prevalence of seropositivity for CMV varies in different geographic areas of
the United States. Most of these donors have latent infections but there is not
currently a test available to distinguish between donors who are infectious and
those who are not. In the absence of CMV-seronegative blood, leukoreduced blood
components are considered an appropriate alternative.
Other infections such as malaria, babesiosis,
and try-panosomiasis may be transmitted by transfusion. There is no specific
testing for malaria in the United States, and exclusion of infected donors is
through the medical his-tory. For other potential infections, local blood
centers often add specific questions related to local infectious risks.
NAT for HIV and HCV was licensed in the United
States in 2003. Investigative NAT testing for West Nile virus, which can be
transmitted by transfusion and transplantation, is currently being conducted
under FDA-IND in the United States. Systems for detecting bacterial
contamination of platelets are now licensed.
Related Topics