Textiles and Dress Designing - Types of Finishes | 12th Textiles and Dress Designing : Chapter 1 : Finishing
Chapter: 12th Textiles and Dress Designing : Chapter 1 : Finishing
Types of Finishes
Types of Finishes
Scouring and bleaching are done prior to other finishes, as they
improve the property of raw fabric for further processing hence they are also
grouped under finishing. Some of the common finishes are discussed below.
1. Scouring
Scouring is the process by which all natural and additive
impurities such as oil, wax, fat and dust. are removed to produce hydrophilic
and clean textile material. It is one of the vital processes of wet processing.
Objectives of Scouring
·
To make the fabric highly hydrophilic
·
To remove impurities such as oils, waxes, gum, husks as nearly as
possible
·
To increase the absorbency of fabric or textile materials without
physical and chemical damage
·
To produce a clean material by adding alkali
·
To prepare the fabric for subsequent process
·
To remove non-cellulosic substance in case of cotton
Scouring can be carried out by two methods:
Saponification
The vegetable oil like glycerides of fatty acids, are present in
the raw fabric. These oils are immiscible with water and hence they are heated
with a solution of sodium hydroxide in water making the oil split up into its
constituents-fatty acid and glycerine. Glycerine is mixable with water easily
and the fatty acids react with sodium hydroxide present in the solution forming
its sodium salt i.e. soap which is also soluble in water. Thus oil is removed
from the fabric.
Emulsification
The wax and non saponifiable oils present in the fabrics are
removed by emulsification. As the waxes and oils are not mixable in water a
normal washing soap is used as an emulsifying agent. The soap makes an emulsion
of the waxes and non saponifiable oils in water and brings them out. The
changes that occur during scouring are as follows :
·
Saponifiable oils and free fatty acids are converted into soaps
·
Pectins and pectoses are converted into soluble salts of pectic
acid
·
Proteins are degraded to simple soluble amino acids or ammonia
·
Mineral matters are mostly dissolved
·
Non-saponifiable oils are emulsified by the soluble soaps
generated from the saponifiable oils
·
Additive stains are removed
·
Residual sizing materials are broken down into soluble products
The chemicals used in scouring are:
1.
Caustic soda - to neutralize acidic materials, to saponify
glycerides (waxes and oils), to solubilise silicates.
2.
Surfactants - to reduce surface tension and minimize interfacial
tension.
3.
Detergents - to emulsify oil, fats, waxes and remove oil -borne
stains.
4.
Chelating agent - to deactivate metal ions.
5.
Sodium silicate - to penetrate and break down lignins
6.
Soda ash - to maintain pH
7.
Solvent - to assist emulsification by dissolving oily materials.
2. Bleaching
Bleach is a chemical that removes colour or whitens a fabric via
oxidation or reduction method. Many types of bleach have strong bactericidal
properties, and are used for disinfecting and sterilizing.
Bleaching is another important pre-treatment next to scouring,
performed on cotton fibres. This treatment is given to decolourize the natural
colouring matter present in the cotton fabrics and impart a pure white colour.
It increases the ability of the textile materials for dyeing and printing by
removing any traces of colour present in it. Normally oxidative bleaching
action is performed in the industries on cotton fibre substrates. Though a
number of bleaching agents are available in the chemical market, few bleaching
agents are used extensively. Calcium hypochlorite (CaOCl), Sodium hypochlorite
(NaOCl) and Hydrogen peroxide (H2O2 ) are the most frequently used bleaching agents
in the conventional cotton processing units.
Hydrogen peroxide is considered a universal bleaching agent, as it
is suitable for all sorts of textiles. It is stable at neutral and value is
near neutral pH. The pH of hydrogen peroxide solution can be modified based on
the suitability. Processing pH of cellulosic is between 9 and 11.5; proteins
2.5 to 6.0 and for synthetics it is near neutral acidic pH.
In order to bleach cotton, the pH of hydrogen peroxide solution is
maintained at around 11. When alkali is added the stability of hydrogen
peroxide is reduced and decomposed at a fast speed. To control the rapid
decomposition a stabilizer is added to the solution. The ingredients added
during peroxide bleaching are normally; hydrogen peroxide (bleaching agent,
1-3% owm), sodium hydroxide or carbonate (bleaching promoters, 0.25 to 1.0%
owm), sodium silicate (buffer or stabilizers, 0.5 to 1.0% owm)
H2O2 ↔ H+ HO2 [Stable]
HO2 ↔ [OH] + (O)[Unstable]
In the alkaline condition the instability of peroxide is continued
by the concentration. The liberation of nascent oxygen is utilized for the
oxidation reaction in a controlled manner by selecting the stabilisers to get
uniform application.

3. Calendaring
It is a mechanical process that finishes the fabric, by passing it
between sets of rollers and applying heat and pressure. The outside of the
rollers can be smoother or engraved to give the perfect finish to the fabric,
the structure of the rollers varies from hardened chromium plated steel to
elastic thermoplastic rollers. By varying the rollers, adding any additional
chemical treatment and temperature, a variety of calendared finishes can be produced
like glazed or moiré fabrics.
Objectives
Calendaring is done for many purposes but the main objectives are:
·
To give softening to the face side of the fabric
·
To increase fabric lustre or glaze
·
To give silk like appearance
·
To close the open threads
·
To decrease the air permeability
·
To increase the fabric clarity
·
To flatten the slubs
·
To modify the fabric surface by embossing
In general a calendaring machine has 2 to 7 rollers with most
common being the 3 bowl rollers. Less number of bowls is used for lightweight
fabrics whereas more numbers of bowls are used for calendaring heavy weight
fabrics. The bowls are made with alternating hard steel and elastic. The
elastic bowl are usually made from compressed paper or compressed cotton,
however a lot of modern calendaring machines are made with Nylon 6 covering.
This provision is given so that there remain resiliency between the two
consecutive rollers and the compression remains uniform. Heating arrangements
are made via steam circulation chamber.

The process parameters that can be controlled during calendaring
are speed of the fabric, speed of the rollers and the surface of rollers.
Different types of calendared effects are: surface glazing, ciré effect, moiré
effect, Schreiner effect and embossing effect.
Different treatments are given to the fabric before calendaring.
For example, to obtain a glazed finish like chintz (polished cotton), the
fabric is first saturated in starch or wax or resin solution and is dried
before calendaring. Starch or wax gives a temporary finish to the fabric but if
the fabric is saturated in resin, it gives a durable glaze.
Calendared fabrics with their glossy or wet look are produced in
the same manner as glazing. The fabrics are coated with a wax or resin before being
calendared with heated rollers. When thermoplastic fibres are used, the fibre
surface that comes in contact with the metal rollers melts and flattens
slightly and produces a highly polished fabric. Examples of calendared fabric
are taffeta, satin or tricot, silk or silk blend fabrics.
Moiré fabrics have characteristic water marked look that is
produced during the calendaring process. The effect is developed using either a
moiré embossing roller or by a high compression calendaring of two layers of
ribbed-base fabric in a single pass. One popular method of preparing moiré
fabric features using rollers that have been engraved with a design. The fabric
is run between the engraved rollers with some sections of the fabric squeezed
to reveal the finished design that has a watery look. This type of application
is often used to create material styles that are ideal for evening gowns,
clutch handbags, and other types of formal apparel and accessories for women.

Schreiner finishes on fabrics produce soft lustre and hand by
flattening the yarns and surface of a fabric through calendaring. The Schreiner
calendar has metal roller engraved with 200-300 fine diagonal lines per inch
that are visible only under a magnifying glass. A Schreiner finish is used on
cotton sateen and table damask to make them more lustrous and on nylon tricot
to increase its cover.
Fabric embossing can also be carried out in the calendaring
machine in which the fabrics can have a pattern imprinted or embossed into it.
The embossed pattern is created by passing the fabric between a heated
embossing roller and a shaped paper roll. The degree of lustre given to the
fabric can be modified by heating the chilled iron roll, by changing the
pressure at the nip, by changing the speed of the machine, by carrying the
moisture percent present in fabric. Various designs can be created by using a
2- bowl embossed roller or 3-bowl embossed roller arrangement.
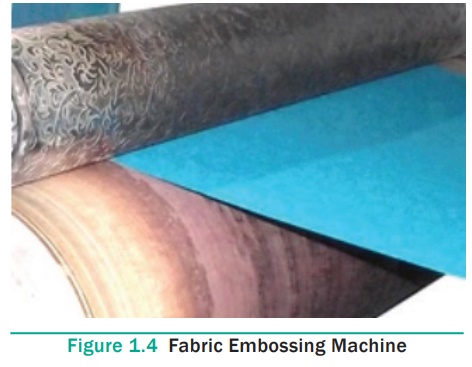
4. Embossing
Embossing is a process that alters the surface of the fabric by
providing a three dimensional or raised effect on selected areas. The embossing
procedure requires the use of two dies: one that is raised and one that is
recessed. When the dies are produced, a die maker engraves the desired design
into several metal plates, which are the embossing dies for use on an embossing
machine. The engraved and the recessed dies fit into each other so that when
the fabric is pressed between them, the raised die forces the fabric into the
recessed die and creates the embossed impression. A specific level of pressure
is applied to the dies in order to squeeze the fibres, which results in a
raised area. The embossed design is permanent if the fabric has a thermoplastic
fibre content or if a resin is used or the fabric is heat set. Embossing is often
used in combination with foil stamping.
5. Sizing or Starching
Size is one of the numerous substances that is applied to a yarn
or fabric to act as a protective filler or glaze. In sizing or starching, the
fabric is immersed in a mixture containing waxes, oils, glycerines and
softeners to control the fabric body by adding stiffness and weight. If the
sizing is resin based and heat set, it is permanent in nature but if the size
is water soluble, it is removed during washing. Gelatin is used on rayon’s
because it is a clear substance that enhances the natural lustre of fibres.

In the weaving process the fabric warp has to undergo several
types of actions like cyclic strain, flexing, abrasion at various loom parts
and inter yarn friction. To reduce the breakage of yarns in the weaving process
the warp yarns are sized before weaving which increases its strength,
abrasion resistance and decreases the yarn hairiness. Different types of water
soluble Polymers Vinyl Alcohol (PVA) called textile sizing agents or chemicals
such as modified starch, Polyvinyl Carboxy Methyl Cellulose (CMC) and acrylates
are used as sizing agents. In order to reduce the abrasiveness of the warp
yarns wax is also added to the sizing liquor. The type of yarn material, the
thickness of the yarn, type of weaving machinery determines the sizing recipe.
The sizing can be done by hand or sizing machine. After the weaving process
gets over the fabric is desized to remove the sizing liquor.
6. Stiffening
Stiffening agents are applied to the cloth to increase the weight
of the fabric, to improve its thickness and lustre. Depending on the end use of
the fabric, some fabrics are needed to be made stiffer and crispier. But, the
effect of these stiffening agents is temporary and once the fabric is washed,
most of the finishes are removed. Stiffening agents such as starches which are
used for finishing of cotton cloth are derived from potato, wheat or corn.
Dextrin’s are used for dyed and printed fabrics as they do not have any undue
effect on the dye or print of the cloth. Natural gums are mainly used in
printing as well as finishing process whereas modified cellulose like resins
are used as stiffening agents.

Acid stiffening is mostly used for fine yarn cotton fabrics as it
gives stiffness as well as transparency to the fabric. It involves rapid
immersion in sulphuric acid, followed by immediate neutralization by sodium
hydroxide. The finish is permanent in nature and is also known as Organdi finish
or Parchmentisation.
7. Softening
Softening of fabric is a very important functional finish which is
required to give a pleasant hand feel to the fabric and impart better
drapability. Fabrics that are harsher and stiffer because of their construction
or due to some prior finishing process are softened by this method. Softening
can be done by either mechanical or chemical process. Simple calendaring
softens hand of the fabric, but is temporary in nature. Silicone compounds are
mostly used as softeners which are durable and require curing. The different
types of softeners are anionic softeners, cationic softeners, non-ionic
softeners, reactive softeners, emulsion softener and silicon softeners. Other
types include emulsified oils and waxes which often result in a semi-durable
finish.

Anionic softeners are not fast to wash. They are compatible with
resin and used as a temporary finish with starch and cationic product.
Example: Sulphonated oils and fatty alcohol sulphates. Non-ionic
softeners have excellent stability against yellowing and are not fast to
dyeing. Cationic softeners are substantive to cellulosic material and
therefore, remain on cloth for few washes. They are compatible with resins.
Reactive softeners are more durable softeners and react chemically with the
(–OH) groups of cellulose. They are more expensive and also toxic in nature.
Emulsion softeners are more popular because it reduces the loss of tear
strength on resin finish and are fast to washing. Silicon softeners are the most
used softeners in recent times. These are the manmade polymers based on the
framework of alternate silicon and oxygen bonds with organic substituent are
attached to silicone.
8. Shearing
Shearing is a process that evens out the length of the pile of a fabric
in a controlled manner. It removes the surface fibres, yarn ends, knots and
similar irregularities and surface flaws with the help of cropping or cutting.
The fabric is passed through a series of tension bars and over an angled
shearing bed which uses blades to cut the protruding fibres. The shearer head
consists of spiral blade which is in contact with a ledger blade. The fabric is
wound helically around a rotating cylinder which moves around spiral blade and
ledger blade. Strong suction is used to remove the cut fibres from the machine.
The distance to move the bed of ledger blade is adjustable and the
height of the pile can be regulated. Shearing may also create a smooth surface
or a patterned or sculptured effect by flattening portions of the pile with an
engraved roller and shearing off the areas that remain erect, and steaming the
fabric to raise the flattened or taller portions. Thus Shearing can be used to
create raised patterns or to smooth the overall nap of a fabric.
Some sheared fabrics are also brushed. Fabrics are brushed to
remove loose fibres, and in some cases, to direct the nap of the surface in a
single direction. Common examples of fabrics with brushed finishes are brushed
corduroy, brushed denim and brushed flannel.


9. Singeing
Singeing is the removal of surface fibres by an intensive flame or
by reflected heat. Singeing refers to burning off. It is an important
pre-treatment process of the fabric and if not done properly results in unclear
prints and patterns. It is often used with shearing to control surface fibres
and particularly fibre blends. Singeing is more intensive than shearing as it
penetrates deeper into the fabric than is possible by shearing, which is
limited to the fabric surface.
Objectives
·
To get a clear and smooth surface
·
To obtain a soil less fabric
·
To print patterns with higher clarity
At first the fabrics are brushed lightly to remove the undesirable fibres and then the fabric is passed over heated copper plates. The flames burns the fibre ends and the singeing area then enters a water bath. The water bath stops any singeing sparks and later the cloth is removed.

10. Napping
It is a mechanical finish in which fibres are raised from the
woven or knitted fabrics by rotating, bristled and wire covered brushes. The
fabric brings out raised fibres all through its surface. The examples of napped
fabrics are cotton flannel, rayon flannel, woollen and worsted napped fabric
like kersey and melton. Napped fabrics have softer handle, better insulation
properties due to more air entrapment. These fabrics are mainly used as
blankets and for winter clothing.

11. Mercerization
It is a finishing treatment of cotton and/or natural fibres
composed by cellulose with a strong caustic alkaline solution (300 g/l) in
order to improve its appearance by making it lustrous. The finish was named
after its discoverer, John Mercer (1791 -1866). He invented a process in which
cotton can be given a lustrous finish resembling silk which was named “mercerization”.
The strong caustic soda on cellulosic material causes the fibres to swell and
simultaneously there is a longitudinal shrinkage in the fibre. The
morphological structure of the fibre gets modified giving it a shinier surface
which is also resistant to wear and washing. Thus, we can say that mercerising
results in the swelling of the cell wall of the cotton fibre which increases
its surface area and reflectance and giving the fibre a softer feel.
Types of Mercerization
There are two types of mercerization:
Tension Mercerization
·
The purpose of tension mercerization is to increase lustre of
Cotton fibres
·
The fibre untwists and swells, lumen becomes rounder in
cross-section and it gains lustre
·
Dye affinity and chemical reactivity increase
·
Fabric becomes stronger and smoother
Slack Mercerization
·
Slack mercerization is not as lustrous as tension mercerization
·
Elongation and recovery properties improves
·
Used for producing comfort stretch garments and fabric bandages,
which need to conform to body shapes.
Mercerisation alters the chemical structure of the cotton fibre.
The structure of the fibre inter-converts from alpha-cellulose to a
thermodynamically more favourable beta-cellulose polymorph.

Process
The mercerizing process involves these three steps:
Step 1: Impregnation of the material in relaxed state with cold caustic solution of
required strength and wet-ability.
Step 2: Stretching while the material is still impregnated in the caustic solution.
Step 3: Washing off the caustic soda from the material while keeping the material still in the
stretched state.
An optional last step in the process is passing the thread over an
open flame; this incinerates stray fibres, improving the fabric’s appearance.
This is known as “gassing the thread” due to the gas burner that is typically
used.
Mercerizing can take place directly on grey cloth, or can also be
done after bleaching. It can be done with or without tension in both cold and
hot conditions. In both cases the mercerised cotton has an increased affinity
for both reactive and direct cotton dyes, water and an increased strength.
Advantages of Mercerization
·
Larger dyeing affinity
·
Larger dimensional stability of the articles
·
Increasing of the lustre
·
Increasing of the tensile strength
·
Better covering of dead cotton
·
Improving touch
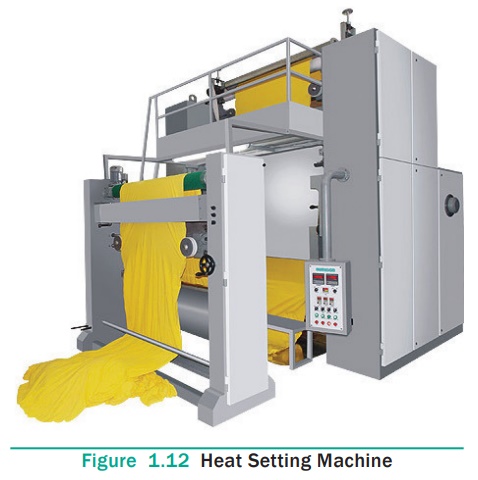
12. Heat Setting
Heat setting is an important part in textile finishing. It is one
of the functional finishes which are carried out mainly on synthetic fabrics.
It eliminates the internal tensions generated during the manufacture of fibre
and fixes the new state by rapidly cooling it. Heat setting fixes the fabrics
in the relaxed state and thus avoids subsequent shrinkage or creasing of
fabric. Presetting of goods make it possible to use higher temperature for
setting without considering the sublimation properties of dyes and also has a
favourable effect on dyeing behaviour and running properties of goods. On the
other hand, post-setting can be combined with some other operations such as
thermasol dyeing or optical brightening of polyester. Post-setting as a final
finish is useful to achieve high dimensional stability, along with desired
handle.
13. Water Proof Finish
Water proof finish gives the resistance of water to the fabric. To
increase the resistance various substance like paraffin, acid, resin, tannin,
drying oils, alum or alumina salt carbonate magnesia are applied on to the
fabric. The number of times the coating is done varies depending upon the
substance used. A water-proof fabric is completely moisture proofed. The fabric
is coated or laminated with a film of natural or synthetic rubber or plastic,
such as vinyl or polyurethane to give proofing effect. Water proof finishes
adversely affect the comfort property of the fabric as they limit the passage
of air and possesses a rather firm and a bad hand feel.

14. Water-Repellent Finish
Water repellent finishes are chemical finishes which resist the
penetration of water into the fabric but permits the passage of moisture or
air. When the fabric becomes very wet the water eventually passes through it.
The yarns are coated with the repellent material like wax which permits the
passage of air and vapour between the interlacing in the fabric. Water and the
other liquid remain on the surface in small bead rather than spreading out and
getting absorbed. The chemicals used are silicones, fluorocarbons, paraffin’s
etc. Some chemicals used for water repellency are also stain repellent. The
combination of fabric finish and structure is important to achieve water
repellent finish because it depends on the surface tension and fabric
penetrability. The combination can make fabric which is stain resistance,
having soft feel and a good drape.
Water repellent finish can be of both durable and non-durable
types. The non-durable repellents are easily removed in laundering or dry
cleaning. They do not provide satisfactory resistance to oily liquids. Durable
repellent finish can be either repellent to water or oil or both. Fluro-carbon
compounds have excellent durability to both dry cleaning and laundering.
15. Flame Retardant Finish
Flame retardant finishes is one of the varieties of functional
finishes. They play an important role on textiles by providing safety and
giving escape time from a potential hazard. When a fire starts flame,
retardants reduce the flame spread and rate of fire development by blocking the
flames access to fuel and hindering future flame propagation. Boric acid/Borax,
Di-ammonium Phosphate and Phosphoric acid, Sulfamic acid and Ammonium Sulfamate
are a few substances used for non -durable flame retardant finishes. The
durable flame retardant finishes include chemicals such as THPC- Tetakis
Hydroxymethyl Phosphonium Chloride and its derivatives, N -Methyldimethyl
Phosphonopropioamide, Phosphonic and Phosphoric Acid and its derivatives.

16. Anti-Microbial Finish
Antimicrobial finishes restrain the disease and decrease the risk
of infection from following injury likes development of bacteria, other aroma
causing germs, damage from perspiration and decay. These finishes are also
called anti- bacteriostatic, germicidal or antiseptic finishes These finishes
are mainly used for clothing that comes in close contact with the skin like
shoe linings, hospital linings and carpeting. Wall covering and upholstery are
also treated with antimicrobial chemicals. It is added to the spinning
solutions in manufacturing fibres.
The most common chemical used for imparting anti -microbial finish
is ziconium peroxide and sometimes an exposure to ethylene oxide gas is also
used. Sutures, bandages and surgical gloves are treated with ethylene oxide
because it is easy to available, low cost, safer and ultimate for medical
products. The sterile environment to be maintained until the package is opened.
Antimicrobial finish process includes gas treatment, chemical treatment and
irradiation treatment.

17. Antistatic Finish
Synthetic fibres of hydrophobic nature are prone to generation of
static charges. This problem is very troublesome while processing the fabric at
high speed in dry state. Static electricity is produced or created when two
non-conducting surfaces such as synthetic textiles rub together. The two
surfaces become oppositely charged and as the rubbing continues an electrical
charge builds up. The wearer can experience the electric shocks and the fabric
tends to cling to the body of the wearer. Antistatic finishes are chemical
substances applied to reduce and eliminate static charge. The chemical
substance used absorbs moisture from the atmosphere and thus reducing the
dryness of the fabric that causes the static charge build up. Anti-static
effective chemicals are largely chemically inert and require thermosol or heat
treatment for fixing. In general thermsolable anti-static agents also have a
good soil release action which is as permanent as the anti-static effect.
Anti-static finishes may also be of polyamide type being curable at moderate
temperatures.
18. Moth Proof Finish
Moth proofing finish is a kind of functional finish given to
textiles to prevent the growth of moths. Moths like silverfish attack fibres
like cotton and wool. Fluorine compounds, napthalene, DDT and paradichloro
benzene are some of the chemicals used for imparting moth proof finishes to
fabrics. They are available in crystal, cake and spray form. Cellulosic fibres
are also treated with boric acid to prevent the rapid growth of mildew and
fungus.

19. Shrink Proofing or Sanforising or Compacting
Controlled residual shrinkage is an important quality parameter
for many fabrics. For example, excessive shrinkage is undesirable for fabrics
to be made into garments. Here, the residual shrinkage should be less than 2%
otherwise the garment will not fit after it is laundered. Mechanical compacting
is one method of reducing residual shrinkage. The process forces yarns closer
together and the fabric becomes thicker and heavier. As a result of this, the
net yardage yield is reduced. A sanforizer is a fabric compactor developed by
Cluett Peabody. The term ‘Sanforized”, is their registered trademark and is
used to market fabrics that meet certain shrinkage specifications. The term
Sanforized is now generally accepted to mean a fabric that has low residual
shrinkage. It is used to describe shrink proofing processes. The process,
consists of a range where the fabric is first moistened with steam, to make it
more pliable, run through a short tenter frame to straighten and smooth out
wrinkles ,through the compressive shrinkage head and then through a Palmer
drying unit to set the fabric.
The key to any compactor is the head where force is applied to
move parallel yarns closer together. More fabric must be fed in than is taken
off. A Sanforizer uses a thick rubber blanket running against a steam heated cylinder
as the compacting force. The thick rubber blanket first goes over a smaller
diameter roll which stretches the convex surface of the blanket. Fabric is
metered onto the stretched blanket and the fabric and blanket together come in
contact with the steam heated cylinder. At this point, the stretched rubber
surface contracts to its original length and then is forced to contract an
additional amount as it forms the concave configuration of the heated drum.
Since the fabric is not elastic, an extra length of fabric is thrust between
the rubber blanket and the heated cylinder. Friction between the rubber blanket
and steel drum force adjacent yarns to move closer together until the unit
length of fabric become equal to the unit length of rubber blanket it rests on.
Heat is created by constantly stretching and relaxing the rubber blanket. The
blanket is cooled by spraying water on it after the fabric exits from the unit.

The degree of shrinkage can be controlled by the thickness of the
blanket. The thicker the blanket, the greater is the stretched length at the
bend. A longer length of fabric will be fed into the compactor causing the
degree of compacting to be greater. To be effective, the degree of compacting
needed should be predetermined ahead of time. This is done by characterizing
the shrinking behaviour of the fabric by laundering. The degree of compacting
should not exceed the degree of shrinking otherwise over-compacting will cause
the fabric to “grow” when relaxed.
20. Soil Release Finish
Textile materials are attracted to dirt or soil. Development of
static charge electricity to hydrophobic textiles makes them prone to soiling.
This is not readily removed during laundering and gets re-deposited on the
fabric. Also, the hydrophobic materials are not wetted properly during
laundering which causes problem with staining.

Soil release finish is one of the functional finishes which work
by making the textile fibres more absorbent or hydrophilic. The hydrophilic
finishes increase the wettability of the fibre and facilitate soil release
during washing. It also prevents soil re-deposition on the fabric. It also
reduces the static charge on the cloth by maintaining moisture on the fabric
surface which is mostly observed in polyester fabrics. They also improve the
antistatic properties, fabric drapability and comfort.
21. Wrinkle Resistant or Crease Resistant Finish
The ability of the fabric to resist the formation of crease or
wrinkle when slightly squeezed is known as ‘crease resistance’ of the fabric.
The ability of a fabric to recover from a definite degree from creasing is
called crease recovery. Finishes to reduce the undue wrinkles on fabric or
garments is called as wrinkle resistance finish. Cotton, rayon and flax are
more susceptible to wrinkles due to the hydrogen bonds of the cellulosic
molecules in their amorphous region. Due to application of heat or moisture,
the hydrogen bond breaks and new hydrogen bond occurs at new dimensions.
Therefore wrinkling can be reduced if the hydrogen bond formation is less.
Resins such as Formaldehyde, DMU (Di-methylol urea), DMEU (Di-methylol ethylene
urea), DMDHEU (Di-methylol di-hydroxyl ethyleneurea), and Modified DMDHEU
(Di-methylol di-hydroxylethylene urea) are mainly used for imparting wrinkle
resistance finish to a fabric.

Related Topics