Chapter: Modern Pharmacology with Clinical Applications: Drugs Used in Neurodegenerative Disorders
Therapy of Parkinsonism
Therapy of Parkinsonism
Since there is no cure for
parkinsonism, the aim of phar-macological therapy is to provide symptomatic
relief. This is obtained through the use of drugs that either in-crease dopaminergic
actions or diminish neuronal out-flow from the striatum. These drugs include
levodopa, which increases brain dopamine levels; dopamine ago-nists, which
directly stimulate dopamine receptors; monoamine oxidase (MAO) inhibitors,
which prevent dopamine metabolism; and anticholinergic agents, which reduce the
excitatory activity within the striatum (Fig 31.3).
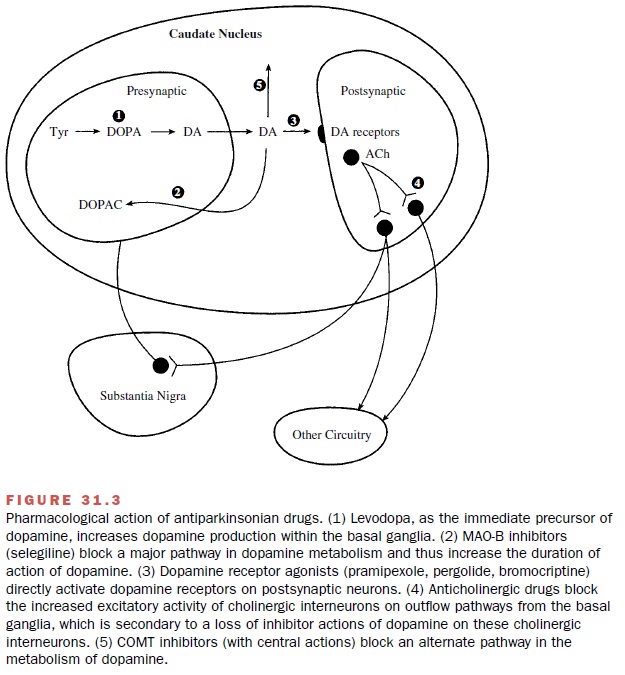
Levodopa and Carbidopa
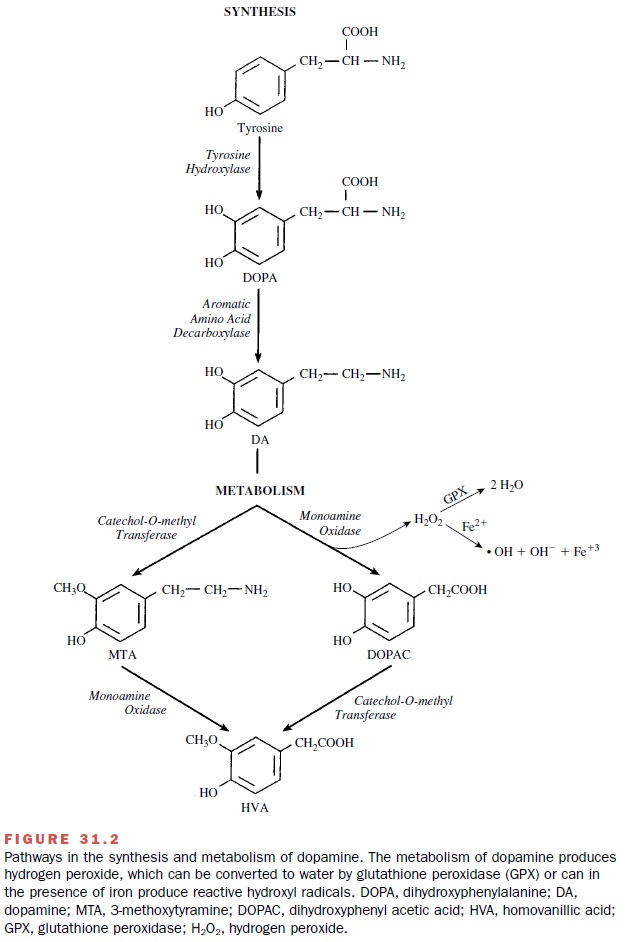
Levodopa (L-DOPA), the most reliable and effective drug used in the treatment of parkinsonism, can be con-sidered a form of replacement therapy. Levodopa is the biochemical precursor of dopamine (Fig. 31.2). It is used to elevate dopamine levels in the neostriatum of parkin-sonian patients. Dopamine itself does not cross the blood-brain barrier and therefore has no CNS effects. However, levodopa, as an amino acid, is transported into the brain by amino acid transport systems, where it is converted to dopamine by the enzyme L-aromatic amino acid decarboxylase.
If levodopa is administered
alone, it is extensively metabolized by L-aromatic amino acid decarboxylase in
the liver, kidney, and gastrointestinal tract. To prevent this peripheral
metabolism, levodopa is coadministered with carbidopa (Sinemet), a peripheral decarboxylase inhibitor. The combination of levodopa with carbidopa
lowers the necessary dose of levodopa and
reduces pe-ripheral side effects associated with its administration.
Levodopa is widely used for
treatment of all types of parkinsonism except those associated with
antipsy-chotic drug therapy. However, as parkinsonism pro-gresses, the duration
of benefit from each dose of levo-dopa may shorten (wearing-off effect). Patients can also develop sudden,
unpredictable fluctuations between mobility and immobility (on-off effect). In a matter of minutes,
a patient enjoying normal or nearly normal mobility may suddenly develop a
severe degree of parkinsonism. These symptoms are likely due to the progression
of the disease and the loss of striatal dopamine nerve terminals.
Other disturbing behaviors that
can be produced by levodopa therapy are the dyskinesias.
These are exces-sive and abnormal choreiform movements of the limbs, hands,
trunk, and tongue. These dyskinesias eventually occur in 40 to 90% of patients
receiving long-term high-dosage levodopa therapy. The mechanism underlying
these abnormal movements is unclear, but it may be re-lated to fluctuating
plasma levels of levodopa and the presence of hypersensitive dopamine
receptors. The dyskinesias can be reduced by lowering the dosage; however, the
symptoms of parkinsonism may then reap-pear. Most patients prefer to tolerate a
certain degree of dyskinesia if their mobility can be improved by levo-dopa
therapy.
The most common peripheral
side effects are anorexia, nausea, and vomiting (likely due to dopamine’s
stimulation of the chemoreceptor trigger zone of the area postrema in the
medulla oblongata).
Orthostatic hypotension may
occur as a result of the pe-ripheral decarboxylation of levodopa and release of
dopamine into the circulation. Cardiac arrhythmias oc-cur in some patients and
are attributed to the stimula-tion of cardiac α- and β-adrenoceptors by dopamine.
Centrally mediated adverse
effects of levodopa ther-apy include vivid dreams, delusions, hallucinations,
con-fusion, and sleep disturbances, especially in the elderly. Certain drugs
can interfere with the clinical effectiveness or exacerbate the adverse
reactions of levodopa therapy. For instance, nonselective MAO inhibitors
(phenelzine, tranylcypromine) should not be administered with lev-odopa, since
the combination can precipitate a life-threatening hypertensive crisis and
hyperpyrexia.The ad-ditive effects of levodopa and adrenomimetic amines
demonstrate that extreme care should be exercised in treating the symptoms of
asthma or emphysema in pa-tients with Parkinson’s disease. Also, levodopa
should not be given to patients with narrow-angle glaucoma, since it can
produce severe mydriasis that would markedly aggravate the glaucoma. Patients
with a history of cardiac arrhythmias or recent cardiac infarction should
receive levodopa only when absolutely necessary. Also, proteins ingested with
meals may produce suffi-cient amounts of amino acids to compete effectively
with levodopa transport both in the gastrointestinal tract and in the brain.
Levodopa therefore should be administered at least 30 minutes before meals.
Dopamine Agonists
Dopamine receptor agonists
are considered by many clinicians as the first approach to therapy. They have a
long duration of action and are less likely to cause dys-kinesias than
levodopa. The rationale for the use of dopamine agonists is that they provide a
means of di-rectly stimulating dopamine receptors and do not de-pend on the
formation of dopamine from levodopa. As monotherapy, the dopamine agonists are
less effective than levodopa but are often used early in the disease to delay
initiation of levodopa therapy. When used as an adjunct to levodopa in advanced
stages, the dopamine receptor agonists may contribute to clinical improve-ment
and reduce levodopa dosage needs.
The four dopamine agonists
used in the United States are bromocriptine (Parlodel), pergolide (Permax),
pramipexole (Mirapex), and ropinirole
(Requip). Bromocriptine, an ergot
derivative, is an agonist at the D2-receptors and a partial D1-antagonist.
Pergolide, also an ergot derivative, is an agonist at both D1- and D2-receptor
subtypes. The more recently introduced noner-got drugs, ropinirole and
pramipexole, are selective ago-nists at D2-receptor sites.
All four exert similar
therapeutic effects and can produce the same adverse effects seen with
levodopa. The differences between the ergot derivatives and the newer agents
reside primarily in their adverse effects and tolerability. Postural
hypotension, nausea, somno-lence, and fatigue are common adverse effects of
bromocriptine and pergolide therapy and can limit the use of these drugs.
Because of these adverse
effects, the drugs are gen-erally first administered at low doses and then the
dose is gradually increased over weeks or months as toler-ance to the adverse
effects develops. These symptoms are generally less frequent and less severe
with pramipexole and ropinirole, which allows for a more rapid achievement of
therapeutic response. Also, be-cause pramipexole and ropinirole are better
tolerated, they are increasingly used as monotherapy.
Selegiline
Another drug used in the
treatment of Parkinson’s dis-ease is selegiline (also known as deprenyl, or Eldepryl). It is an irreversible
inhibitor of MAO-B, an important enzyme in the metabolism of dopamine (Fig.
33.2). Blockade of dopamine metabolism makes more dopamine available for
stimulation of its receptors. Selegiline, as monotherapy, may be effective in
the newly diagnosed patient with parkinsonism because its pharmacological
effect enhances the actions of endoge-nous dopamine.
Selegiline is also used in
conjunction with levodopa– carbidopa in later-stage parkinsonism to reduce
lev-odopa dosage requirements and to minimize or delay the onset of dyskinesias
and motor fluctuations that usually accompany long-term treatment with
levodopa. It has also been proposed that selegiline may slow the progression of
the disease by reducing the formation of toxic free radicals produced during
the metabolism of dopamine (Fig.31.2). However, any neuroprotective ef-fect of
selegiline in parkinsonian patients remains to be established.
Most of the adverse reactions
to selegiline are re-lated to actions of increased levels of dopamine, as
dis-cussed earlier. At recommended doses, and unlike the nonselective MAO
inhibitors used in the treatment of depression, selegiline has little effect on
MAO-A and therefore generally does not cause the hypertension as-sociated with
the ingestion of tyramine-enriched foods . However, at doses higher than those
usually recommended, MAO-A may be inhibited, which increases the risk of a
tyramine reaction.
Selegiline should not be
coadministered with tricyclic antidepressants or selective serotonin uptake
inhibitors because of the possibility of a severe adverse drug reac-tion (e.g.,
hyperpyrexia, agitation, delirium, coma).
Anticholinergic Drugs
Before the introduction of
levodopa, the belladonna alkaloids (e.g., atropine and
scopolamine) were the primary agents used in the treatment of parkinsonism. The
belladonna alkaloids have been replaced by anti-cholinergic agents with more
selective central nervous system (CNS) effects. Trihexyphenidyl (Artane), ben-ztropine mesylate (Cogentin), biperiden (Akineton), and procyclidine (Kemadrin) are useful in most types of
parkinsonism.
The efficacy of
anticholinergic drugs in parkinsonism is likely due to the ability to block
muscarinic receptors in the striatum. In the absence of the inhibitory action
of dopamine, the actions of the intrastriatal cholinergic in-terneurons are
unopposed, yielding enhanced stimula-tion of muscarinic receptors. Blockade of
these recep-tors reduces striatal activity. The
muscarinic antagonists exert only
modest antiparkinsonian actions and thus are most commonly used during the
early stages of the dis-ease or as an adjunct to levodopa therapy.
Of the drugs used for
treating parkinsonism, the an-ticholinergics are the only class that can
provide benefit in the treatment of the drug-induced parkinsonism seen with
antipsychotic therapy. This is because the blockade of dopamine receptors by
the antipsychotics leads to in-creased activity of the striatal neurons. Blockade
of the muscarinic receptors reduces this excitatory activity.
The adverse effects of the
anticholinergic drugs are due to their antimuscarinic effects in other systems
(e.g., cycloplegia, dry mouth, urinary retention, and con-stipation).
Confusion, delirium, and hallucinations may occur at higher doses.
The antihistamine
diphenhydramine (Benadryl), be-cause
it has anticholinergic properties, is used for mild parkinsonism and with the
elderly, who may not be able to tolerate the more potent anticholinergics,
levodopa, or the dopamine agonists.
Amantadine
Amantadine was originally
introduced as an antiviral compound , but it is modestly effective in treating
symptoms of parkinsonism. It is useful in the early stages of parkinsonism or
as an adjunct to levo-dopa therapy. Its mechanism of action in parkinsonism is
not clear, but amantadine may affect dopamine release and reuptake. Additional
sites of action may include antagonism at muscarinic and N-methyl-D-aspartate (NMDA) receptors. Adverse effects include
nausea, dizziness, insomnia, confusion, hallucinations, ankle edema, and livedo
reticularis. Amantadine and the anticholinergics may exert additive effects on
men-tal functioning.
Catechol-O-Methyl
Transferase Inhibitors
A recently introduced class
of drugs for the treatment of parkinsonism is the catechol-O-methyl transferase (COMT)
inhibitors. COMT metabolizes catechol
com-pounds, including dopamine and levodopa, producing the inactive compound 3-O-methyl DOPA. The rationale for the use
of COMT inhibitors is analogous to that for carbidopa; that is, since COMT is
present in the periphery as well as in the CNS, inhibition of peripheral COMT
results in an increase in the plasma half-life of levodopa, thereby making more
drug available for transfer to the brain. Additionally, com-pounds that block
COMT in the CNS also prolong the brain concentration of levodopa.
The two COMT inhibitors in
clinical use are tol-capone (Tasmar)
and entacapone (Comtan). They are
used in combination with levodopa–carbidopa. In pa-tients with motor
fluctuations, they increase the “on” time. Adverse effects are similar to those
observed with levodopa–carbidopa alone. Tolcapone therapy can cause fatal
hepatotoxicity and so should be used only in patients who do not respond to
other therapies. Patients taking tolcapone require close monitoring of liver
en-zymes for signs of hepatic changes.
Nonpharmacological Approaches to the Treatment of Parkinsonism
Additional approaches to the
treatment of Parkinson’s disease include surgical procedures, brain
stimulation, and transplantation of dopaminergic cells. In general, surgical
procedures are reserved for patients who are refractive to levodopa or who have
profound dyskine-sias or fluctuations in response to levodopa. Tremor can be abolished
by ablation of the ventral intermediate nu-cleus of the thalamus. Dyskinesias
can be effectively controlled by ablation of the posteroventral portion of the
globus pallidus. Brain stimulation appears to be a promising technique.
High-frequency electrical stimula-tion of the thalamus, subthalamic nucleus, or
globus pal-lidus can improve various symptoms of parkinsonism and reduce
levodopa dosage.
A potentially promising,
although very controver-sial, approach to the treatment of Parkinson’s disease is
replacement of dopaminergic neurons. The grafting of fetal substantia nigra
tissue, which contains the dop-amine neurons, into the striatum of parkinsonian
pa-tients has been modestly successful. The procedure will remain experimental,
however, until the many practical problems and ethical issues associated with
the use of fetal tissue are resolved. The discovery of pluripotent stem cells
is also being viewed as a possible way of de-veloping dopamine neurons for
transplant purposes.
Related Topics