Chapter: Basic & Clinical Pharmacology : Rational Prescribing & Prescription Writing
The Prescription
THE PRESCRIPTION
Although a
prescription can be written on any piece of paper (as long as all of the legal
elements are present), it usually takes a specific form. A typical printed
prescription form for outpatients is shown in Figure 65–1.
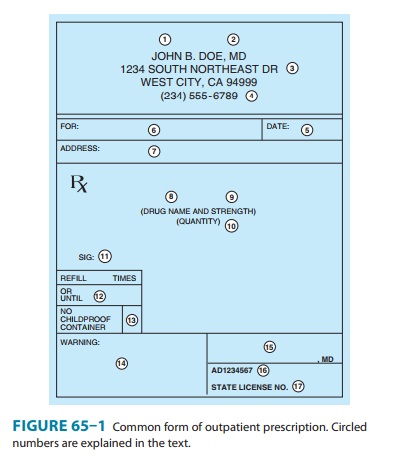
In the hospital
setting, drugs are prescribed on a particular page of the patient’s hospital
chart called the physician’s order
sheet(POS) or chart order. The
contents of that prescription arespecified in the medical staff rules by the
hospital’s Pharmacy and Therapeutics Committee. The patient’s name is typed or
written on the form; therefore, the orders consist of the name and strength of
the medication, the dose, the route and frequency of adminis-tration, the date,
other pertinent information, and the signature of the prescriber. If the
duration of therapy or the number of doses is not specified (which is often the
case), the medication is contin-ued until the prescriber discontinues the order
or until it is termi-nated as a matter of policy routine, eg, a stop-order
policy.
A typical chart order
might be as follows:
11/15/08
10:30 a.m.
(1)
Ampicillin 500 mg IV
q6h × 5 days
(2) Aspirin 0.6 g per rectum q6h prn temp over 101 [Signed]
Janet B. Doe, MD
Thus, the elements of
the hospital chart order are equivalent to the central elements (5, 8–11, 15)
of the outpatient prescription.
Elements of the Prescription
The first four elements
(see circled numerals in Figure 65–1) of the outpatient prescription establish
the identity of the prescriber: name, license classification (ie, professional
degree), address, and office telephone number. Before dispensing a
prescription, the pharmacist must establish the prescriber’s bona fides and
should be able to contact the prescriber by telephone if any questions arise.
Element [5] is the date on which the prescription was writ-ten. It should be
near the top of the prescription form or at the beginning (left margin) of the
chart order. Since the order has legal significance and usually has some
temporal relationship to the date of the patient-prescriber interview, a
pharmacist should refuse to fill a prescription without verification by telephone
if too much time has elapsed since its writing.Elements [6] and [7] identify
the patient by name and address.The patient’s name and full address should be
clearly spelled out.
The body of the
prescription contains the elements [8] to [11] that specify the medication, the
strength and quantity to be dispensed, the dosage, and complete directions for
use. When writing the drug name (element [8]), either the brand name
(proprietary name) or the generic name (nonproprietary name) may be used.
Reasons for using one or the other are discussed below. The strength of the
medication [9] should be written in metric units. However, the prescriber
should be familiar with bothsystems now in use: metric and apothecary. For
practical purposes, the following approximate conversions are useful:
1 grain (gr) = 0.065 grams
(g), often rounded
to 60 milligrams (mg)
15 gr = 1 g
1 ounce (oz) by volume = 30 milliliters (mL)
1 teaspoonful (tsp) = 5 mL
1 tablespoonful (tbsp) = 15 mL
1 quart (qt) = 1000 mL
1 minim = 1 drop (gtt)
20 drops = 1 mL
2.2 pounds (lb) = 1
kilogram (kg)
The
strength of a solution is usually expressed as the quantity of solute in
sufficient solvent to make 100 mL; for instance, 20% potassium chloride
solution is 20 grams of KCl per deciliter (g/dL) of final solution. Both the
concentration and the volume should be explicitly written out.
The
quantity of medication prescribed should reflect the anticipated duration of
therapy, the cost, the need for continued contact with the clinic or physician,
the potential for abuse, and the potential for toxicity or overdose.
Consideration should be given also to the standard sizes in which the product
is available and whether this is the initial prescription of the drug or a
repeat prescription or refill. If 10 days of therapy are required to
effec-tively cure a streptococcal infection, an appropriate quantity for the
full course should be prescribed. Birth control pills are often prescribed for
1 year or until the next examination is due; how-ever, some patients may not be
able to afford a year’s supply at one time; therefore, a 3-month supply might
be ordered, with refill instructions to renew three times or for 1 year
(element [12]). Some third-party (insurance) plans limit the amount of medicine
that can be dispensed—often to only a month’s supply. Finally, when first
prescribing medications that are to be used for the treat-ment of a chronic
disease, the initial quantity should be small, with refills for larger
quantities. The purpose of beginning treat-ment with a small quantity of drug
is to reduce the cost if the patient cannot tolerate it. Once it is determined
that intolerance is not a problem, a larger quantity purchased less frequently
is sometimes less expensive.
The directions for use
(element [11]) must be both drug-specific and patient-specific. The simpler the
directions, the better; and the fewer the number of doses (and drugs) per day,
the better. Patient noncompliance (also known as nonadherence, failure to
adhere to the drug regimen) is a major cause of treatment failure. To help
patients remember to take their medications, prescribers often give an
instruction that medications be taken at or around mealtimes and at bedtime.
However, it is important to inquire about the patient’s eating habits and other
lifestyle patterns, because many patients do not eat three regularly spaced
meals a day.
The
instructions on how and when to take medications, the duration of therapy, and
the purpose of the medication must be explained to each patient both by the
prescriber and by the phar-macist. (Neither should assume that the other will
do it.)
Furthermore, the drug
name, the purpose for which it is given, and the duration of therapy should be
written on each label so that the drug may be identified easily in case of
overdose. An instruction to “take as directed” may save the time it takes to
write the orders out but often leads to noncompliance, patient confusion, and
medica-tion error. The directions for use must be clear and concise to prevent
toxicity and to obtain the greatest benefits from therapy.
Although directions
for use are no longer written in Latin, many Latin apothecary abbreviations
(and some others included below) are still in use. Knowledge of these
abbreviations is essential for the dispensing pharmacist and often useful for
the prescriber. Some of the abbreviations still used are listed in Table 65–1.
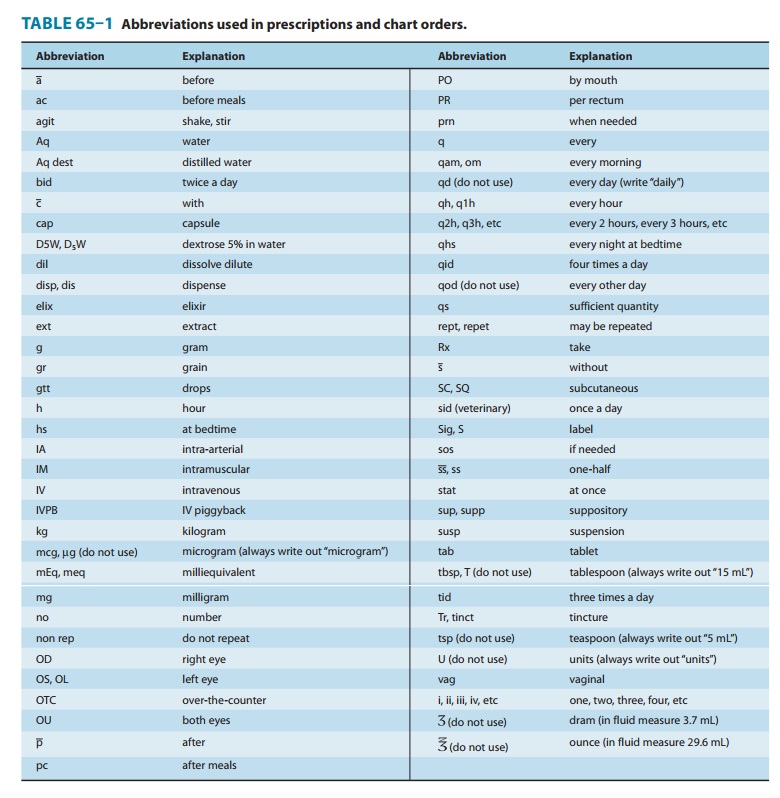
Note: It is always
safer to write out the direction without abbreviating.
Elements [12] to [14]
of the prescription include refill infor-mation, waiver of the requirement for
childproof containers, and additional labeling instructions (eg, warnings such
as “may cause drowsiness,” “do not drink alcohol”). Pharmacists put the name of
the medication on the label unless directed otherwise by the pre-scriber, and
some medications have the name of the drug stamped or imprinted on the tablet
or capsule. Pharmacists must place the expiration date for the drug on the
label. If the patient or pre-scriber does not request waiver of childproof containers,
the phar-macist or dispenser must place the medication in such a container.
Pharmacists may not refill a prescription medication without authorization from
the prescriber. Prescribers may grant authori-zation to renew prescriptions at
the time of writing the prescrip-tion or over the telephone. Elements [15] to
[17] are the prescriber’s signature and other identification data.
Related Topics