Chapter: Pharmaceutical Drug Analysis: Tetrazolium Assay of Steroids
Tetrazolium Assay of Steroids: Theory
THEORY
The oxidation of the α-ketol
moiety present in the steroid under examination and the subsequent reduction of
triphenyltetrazolium chloride to the corresponding triphenylformazan are
depicted in the following reaction :
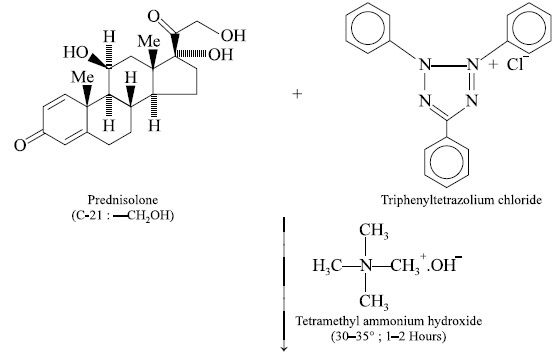
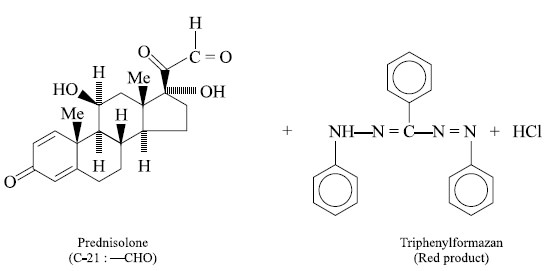
The triphenyltetrazolium chloride ring undergoes
cleavage, as shown by the dotted line, and 2H-atoms are given out by the
steroid prednisolone in being converted from C-21, —CH2OH to C-21,
—CHO function ; one of the H-atoms from above is utilized in the formation of
the open-chain compound i.e.,
triphenylformazan derivative ; whereas, the second H-atom abstracts the Cl–
ion as a mole of HCl. The above interaction is of a quantitative nature.
However, it is pertinent to mention here that certain
steroids esterified at C-21 position, such as : hydrocortisone acetate,
methylprednisolone acetate are duly hydrolyzed in the alkaline medium to give
rise to the corresponding free C-21 hydroxy steroids and hence, may also be
assayed by adopting the same procedure.
Precautions :
All these assays are to be
carried out strictly in the absence of light and atmospheric oxygen to get optimum results.
Related Topics