Chapter: Modern Pharmacology with Clinical Applications: Tetracyclines, Chloramphenicol, Macrolides, and Lincosamides
Tetracyclines
TETRACYCLINES
Structure and Mechanism of Action
Although all tetracyclines
have a similar mechanism of action, they have different chemical structures and
are produced by different species of Streptomyces.
In addi-tion, structural analogues of these compounds have been synthesized to
improve pharmacokinetic proper-ties and antimicrobial activity. While several
biological processes in the bacterial cells are modified by the tetra-cyclines,
their primary mode of action is
inhibition of protein synthesis. Tetracyclines
bind to the 30S ribosome and thereby
prevent the binding of aminoacyl transfer RNA (tRNA) to the A site (acceptor
site) on the 50S ri-bosomal unit. The tetracyclines affect both eukaryotic and
prokaryotic cells but are selectively
toxic for bacte-ria, because they readily penetrate microbial membranes and
accumulate in the cytoplasm through an energy-dependent tetracycline transport
system that is absent from mammalian cells.
Resistance is related largely
to changes in cell per-meability and a resultant decreased accumulation of drug
due to increased efflux from the cell by an energy-dependent mechanism. Other
mechanisms, such as pro-duction of a protein that alters the interaction of
tetra-cycline with the ribosome and enzymatic inactivation of the drug, have
been reported.
Antibacterial Spectrum
The tetracyclines display
broad-spectrum activity and are effective against both gram-positive and
gram-negative bacteria, including Rickettsia,
Coxiella, Mycoplasma, and Chlamydia spp..
Tetracycline resistance has increased among
pneumococci and gonococci, which limits their use in the treatment of
infections caused by these organisms.
Although several congeners of
the tetracyclines are available, they all have a similar spectrum of in vitro
ac-tivity. Minocycline is somewhat more active and oxytet-racycline and
tetracycline are somewhat less active than other members of this group.
Absorption, Distribution, Metabolism, and Excretion
These antibiotics are
partially absorbed from the stom-ach and upper gastrointestinal tract. Food impairs ab-sorption of all
tetracyclines except doxycycline and minocycline. Absorption of doxycycline
and minocy-cline is improved with food. Since the tetracyclines form insoluble
chelates with calcium (such as are found in many antacids), magnesium, and
other metal ions, their simultaneous
administration with milk (calcium), mag-nesium hydroxide, aluminum hydroxide,
or iron will in-terfere with absorption. Because some of the tetracy-clines are
not completely absorbed, any drug remaining in the intestine may inhibit
sensitive intestinal microor-ganisms and alter the normal intestinal flora.
The tetracyclines are
distributed throughout body tissues and fluids in concentrations that reflect
the lipid solubility of each individual agent. Minocycline and doxycycline are
the most lipid soluble, while oxytetracy-cline is the least lipid soluble. The
tetracyclines pene-trate (but somewhat unpredictably) the uninflamed meninges
and cross the placental barrier. Peak serum levels are reached approximately 2
hours after oral ad-ministration; cerebrospinal fluid (CSF) levels are only
one-fourth those of plasma.
The various congeners differ
in their half-lives and their protein binding ability (Table 47.1). Significant
dif-ferences in serum half-life allow the grouping of the tetracyclines into
subclasses: short acting
(tetracycline, chlortetracycline, and oxytetracycline), intermediate act-ing (demeclocycline and methacycline), and long acting (minocycline and
doxycycline).
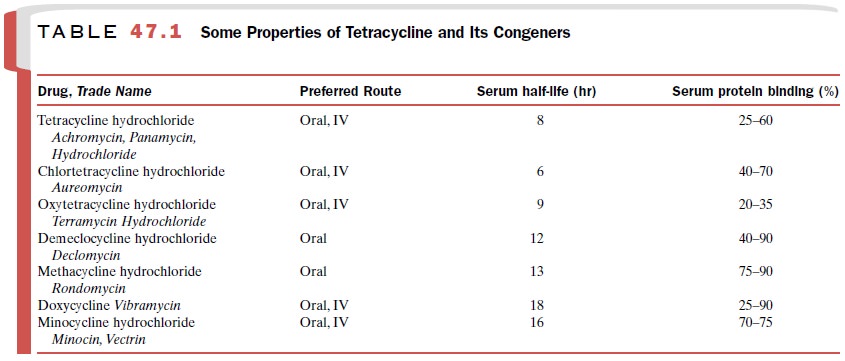
The tetracyclines are
metabolized in the liver and are concentrated in the bile. Bile concentrations
can be up to five times those of the plasma. Doxycycline, minocycline, and
chlortetracycline are excreted prima-
rily in the feces. The other
tetracyclines are eliminated primarily in the urine by glomerular filtration.
Obvi-ously, these tetracyclines have greater urinary antibac-terial activity
than those (e.g., doxycycline) that are ex-creted by nonrenal mechanisms.
Clinical Uses
There is little difference in clinical response among the various
tetracyclines. The selection of an agent, therefore, is based on tolerance, ease of administration, and cost. The
restriction of their use in pregnancy and in patients under the age of 8 years
applies to all preparations.
Two tetracyclines have
sufficiently distinctive fea-tures to warrant separate mention. Doxycycline,
with its longer half-life and lack of nephrotoxicity, is a popular choice for
patients with preexisting renal disease or those who are at risk for developing
renal insufficiency. The lack of nephrotoxicity is related mainly to biliary
excretion, which is the primary route of doxycycline elimination. Doxycycline is the preferred parenteral tetracycline. Doxycycline is a potential
first-line agent in the prophylaxis
of anthrax after exposure. Doxycycline is the treatment of choice for the
primary stage of Lyme disease in adults and children older than 8 years.
Minocycline is an effective
alternative to rifampin for eradication of meningococci, including
sulfonamide-resistant strains, from the nasopharynx. However, the high
incidence of dose-related vestibular side effects renders it less acceptable.
Although minocycline has good in vitro activity against Nocardia spp., further studies are necessary to confirm its
clinical efficacy.
The tetracyclines are still the drugs of choice for treatment of cholera, diseases caused by Rickettsia and Coxiella, granuloma inguinale, relapsing fever, the chlamydial diseases (trachoma, lymphogranuloma venereum, and psittacosis), and nonspecific urethritis.
They are also effective in the treatment of
brucellosis, tularemia, and infections caused by Pasteurella and Mycoplasma spp.,
although other agents may be equally effective.
Tetracyclines are clinically effective in acne because of their antioxidant
effect on the degranulated neutrophils in the comedone acidic contents (in
which long-term low-dose therapy is popular). Mild to moder-ate attacks of
pelvic inflammatory disease often re-spond to tetracycline, probably as a
result of the drug’s action on anaerobic bacteria and chlamydia.
Tetracyclines no longer can
be entirely relied on in the treatment of streptococcal infections; up to 40%
of Streptococcus pyogenes and 10% of Streptococcus pneu-moniae are
resistant.
Adverse Effects
Oral administration can cause
nausea, vomiting, epigas-tric burning, stomatitis, and glossitis, and an
intravenous injection can cause phlebitis. When given over long pe-riods, use
of these agents can result in a negative nitro-gen balance, which may lead to
elevated blood urea ni-trogen. Hepatotoxicity occurs infrequently but is
particularly severe during pregnancy, when the combi-nation of uremia and
increasing jaundice can be fatal. In addition, these antibiotics are
occasionally nephrotoxic and should not be administered with other potentially
nephrotoxic drugs. Staining of both the deciduous and permanent teeth and
retardation of bone growth can occur if tetracyclines are administered after
the fourth month of gestation or if they are given to children less than 8
years of age.
Photosensitivity, observed as
abnormal sunburn reac-tion, is particularly associated with demeclocycline and
doxycycline administration. Superinfection may result in oral, anogenital, and
intestinal Candida albicans
infec-tions, whereas Staphylococcus
aureus or Clostridium dif-ficile overgrowth
may cause enterocolitis. Minocycline can
produce vertigo.
Minocycline is frequently
used in the treatment of chronic facial dermatoses. Increased usage has
resulted in local skin pigmentation, particularly at sites of previ-ous tissue
trauma that is unrelated to the photosensiti-zation phenomenon characteristic
of this class of drug. This effect does not appear to be dose dependent and
usually resolves in months to years following drug dis-continuation.
Other significant side
effects of minocycline may make it unsuitable for some light-skinned patients.
In particular, dark bone pigmentation is severe enough to be visible through
the mucosae of the alveolar ridges in the mouth and other areas where bone
directly adheres to skin (black bone disease). Thyroid staining is visible
through the overlying skin of the neck but does not af-fect the endocrine
function of the gland.
Pulmonary eosinophilic
syndrome, characterized by extreme hypoxemia, eosinophilia, interstitial
pneu-monitis, hilar lymphadenopathy, and pleural effusions, can be severe and
can occur with as little as 7 to 9 days of therapy with the tetracyclines. In
severe cases steroid therapy is required, but the outcome following drug
dis-continuation is nearly always good.
Pseudotumor cerebri is
another potential complica-tion of chronic use of these agents, particularly in
indi-viduals treated for severe cystic acne with simultaneous use of
isotretinoin. This complication can be induced within several days of
initiation of therapy and usually resolves with cessation of treatment.
Chronic use always
predisposes to the development of fungal esophagitis, which may be so severe as
to re-quire treatment with antifungal therapy. Prompt recog-nition of dysphagia
and cessation of treatment are usu-ally curative.
Related Topics