Chemistry Laboratory Practical Experiment - Testing the solubility of the salt | 10th Science : Chemistry Practicals
Chapter: 10th Science : Chemistry Practicals
Testing the solubility of the salt
TESTING THE SOLUBILITY OF THE SALT
Aim:
To test
the solubility of the given salt based on the saturation and un saturation of
the solution at a given temperature.
Principle:
A
solution in which no more solute can be dissolved in the solvent at a given
temperature is called saturated solution. If the solvent can dissolve more
solute than what is present, the solution is called unsaturated solution.
Materials Required:
A 250 ml
beaker, a Stirrer, sufficient quantity of distilled water, 100 ml measuring
jar, table salt in three packets weighing as 25g, 11g, and 1g.
Procedure:
In a
250ml beaker ,pour 100 ml water using measuring jar. To this water add table
salt (25 g) from first packet. stir the content very well. Add the next packet
containing 11 g salt followed by constant stirring . Now add the third packet
containing 1 g salt . Record your observations.
Observation:
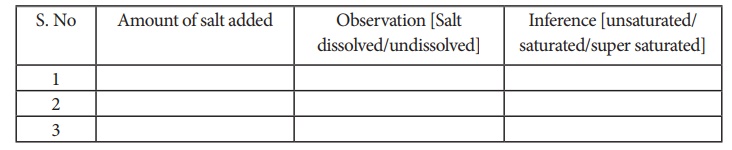
Result:
From the
above observation, it is inferred that the amount of salt required for
saturation is _______ g
Related Topics