Chemistry Laboratory Practical Experiment - Identification of the dissolution of the given salt whether it is exothermic or endothermic | 10th Science : Chemistry Practicals
Chapter: 10th Science : Chemistry Practicals
Identification of the dissolution of the given salt whether it is exothermic or endothermic
IDENTIFY THE DISSOLUTION OF THE GIVEN SALT WHETHER
IT IS EXOTHERMIC OR ENDOTHERMIC.
Aim:
To test
the dissolution of given salt is exothermic or endothermic
Principle:
If the
reaction or process liberates the heat, then it is called exothermic.
If the
reaction or process absorbs the heat, then it is called endothermic
Apparatus required:
Two
beakers, Thermometer, stirrer ,weighed amount of two samples.
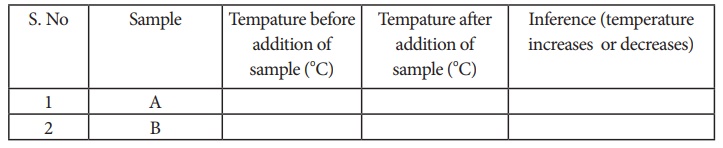
Procedure:
Take 50ml
of water in two beakers and label them as A and B. Note the temperature of the
water from beaker A and B. Then, add 5g of sample A into the beaker A and stir
well until it dissolve completely. Record final temperature of the solution.
Now, repeat the same for the sample B. Record the observation.
Observation:
Result:
From the
inferences made
The
dissolution of sample A is ____________________(Exothermic or endothermic)
The
dissolution of sample B is ____________________(Exothermic or endothermic)
Note:
Sodium
hydroxide, ammonium nitrate, glucose, calcium oxide etc. may be given as the
sample.
Related Topics