Chapter: Modern Pharmacology with Clinical Applications: Synthetic Organic Antimicrobials: Sulfonamides, Trimethoprim, Nitrofurans, Quinolones, Methenamine
Sulfonamides
SULFONAMIDES
Chemistry, Structure, and Function
The sulfonamides are a large
group of compounds that are structural analogues of p-aminobenzoic acid (PABA). They differ primarily in the
substituents on ei-ther the amido group (SO2-NH-R) or the amino
group (-NH2) of the sulfanilamide nucleus. Substitutions on the
sulfonamide group modify the drug’s solubility charac-teristics, resulting in
congeners with different rates of ab-sorption and excretion. One group of
sulfonamides re-mains largely unabsorbed in the gastrointestinal (GI)
tract following oral administration.
Sulfadiazine, for ex-ample, produces changes only on local gut bacterial flora
and finds wide use in presurgical bowel sterilization. Other sulfonamides, such
as sulfisoxazole, are rapidly absorbed and highly soluble, and they undergo
rapid uri-nary excretion, mainly in the unaltered form. A third group are
rapidly absorbed and slowly excreted and maintain adequate blood levels for up
to 24 hours (e.g., sulfamethoxazole). These drugs are useful in treating
chronic urinary infections. Finally, some sulfonamides (e.g., sulfacetamide and
sulfadiazine [silver salt]) are de-signed for topical use such as in infection
of the eye and in burn patients.
Mechanism of Action and Resistance
Both sulfonamides and
trimethoprim (not a sulfon-amide) sequentially interfere with folic acid
synthesis by bacteria. Folic acid functions as a coenzyme in the transfer of
one-carbon units required for the synthesis of thymidine, purines, and some
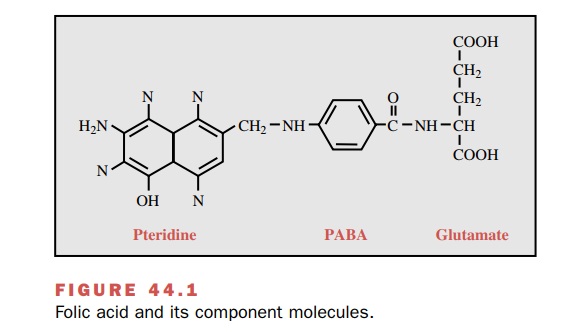
amino acids and con-sists of three components: a pteridine moiety, PABA, and
glutamate (Fig. 44.1). The sulfonamides, as struc-tural analogues,
competitively block PABA incorpora-tion; sulfonamides inhibit the enzyme
dihydropteroate synthase, which is necessary for PABA to be incorpo-rated into
dihydropteroic acid, an intermediate com-pound in the formation of folinic
acid. Since the sulfon-amides reversibly
block the synthesis of folic acid, they
are bacteriostatic drugs. Humans cannot
synthesize folic acid and must acquire it in the diet; thus, the sulfon-amides
selectively inhibit microbial growth.
Resistance to the
sulfonamides can be the result of decreased bacterial permeability to the drug,
increased production of PABA, or production of an altered dihy-dropteroate
synthetase that exhibits low affinity for sul-fonamides. The latter mechanism
of resistance is plas-mid mediated. Active efflux of the sulfonamides has also
been reported to play a role in resistance. The in-hibitory effect of the
sulfonamides also can be reversed by the presence of pus, tissue fluids, and
drugs that con-tain releasable PABA.
Antibacterial Spectrum and Resistance
The sulfonamides are broad-spectrum antimicrobials that are effective against gram-positive and some gram-negative organisms of the Enterobacteriaceae. There is good activity against Escherichia coli, moderate activity against Proteus mirabilis and Enterobacter spp.; poor activity against indole-positive Proteus and Klebsiella spp., and no inhibitory activity against Pseudomonas aeruginosa and Serratia spp. They are also effective against Chlamydia spp., but superior drugs are now available. Sulfonamides are used in treating infections caused by Toxoplasma gondii and occasionally chloro-quine-resistant Plasmodium falciparum.
Resistance occurs as the
result of one or more alter-ations in the cellular metabolism of the bacteria;
both mutation and plasmid-mediated resistance occurs. These changes, which can
be irreversible, include alter-ations in the physical or enzymatic
characteristics of the enzyme or enzymes that metabolize PABA and partici-pate
in the cellular synthesis of tetrahydrofolic acid. The appearance of
alternative pathways for PABA synthesis within the bacteria or the development
of an increased capacity to inactivate or eliminate the sulfonamide also may
contribute to bacterial cell resistance. Bacteria that can use preformed folate
are not inhibited by sulfon-amides.
Pharmacokinetic Properties
Absorption
Sulfonamides are usually
given orally, although the sol-uble sodium salts can be given parenterally, a
route that is infrequently used. Except for compounds designed for local gut
effects, the sulfonamides are rapidly ab-sorbed from the intestinal tract, primarily
from the small intestine. They can usually be found in serum and urine within
30 minutes after ingestion. Peak serum lev-els are obtained in 2 to 6 hours;
urine levels can reach above 500 μg/mL. Although absorption can occur via other
routes (e.g., burned and/or abraded skin, stom-ach), the amounts absorbed are
usually low and unpre-dictable. A burn area larger than 20% of total body
sur-face can absorb enough drug to result in toxicity, especially if
accompanied by renal dysfunction.
Distribution
Systemically absorbed
sulfonamides readily distribute throughout body fluids. They pass the placental
barrier and enter the cerebrospinal fluid (CSF) even in the ab-sence of
inflammation. The degree of protein binding, the half-life, and the drug’s solubility
in urine will vary considerably from one sulfonamide to another. Half-lives
range from 2.5 to 17 hours, the latter exhibited by sulfadiazine. Sulfadiazine
and sulfacetamide tend to have lower protein binding (about 20–30%) than the
other major systemic sulfonamides, whose binding ranges from 80 to 90% (e.g.,
sulfamethoxazole, sulfisox-azole). The effects of high protein binding by a
sulfon-amide become almost negligible in body fluids with a paucity of protein
(e.g., synovial, peritoneal, ocular); thus, the drug in these sites is
primarily in the active un-bound form. Most drugs with protein binding above
30% do not cross the placenta; while this reduces toxic potential, it
concomitantly lowers drug antibacterial activity.
Metabolism and Excretion
The sulfonamides are degraded
in the liver by acetyla-tion and oxidation; metabolites have reduced
bacterio-logical activity. The parent compound and the metabo-lites are
excreted in the urine, primarily by glomerular filtration followed by tubular
reabsorption. Some sul-fonamides exhibit diurnal variations in excretion, being
three times greater at night than during the day.
Clinical Uses
Sulfonamides have a long
record of successful use in the treatment of a wide range of both gram-positive
and gram-negative bacterial infections. They are also active against some of
the less frequently encountered in-fections, such as leprosy, malaria,
toxoplasmosis, and nocardiosis. Current indications are more limited,
espe-cially to the treatment of urinary tract and ear infec-tions, because of
frequently encountered resistance and the availability of better and safer
agents for infections such as shigellosis, salmonellosis, and meningococcal
meningitis. In contrast, the growth of rickettsial organ-isms is actually stimulated.
Acute uncomplicated urinary
tract infections caused by E. coli
and other pathogens generally respond promptly to one of the short-acting
sulfonamides. Recurrent urinary tract infections (UTIs), when related to some
structural abnormality in the tract, are fre-quently caused by
sulfonamide-resistant bacteria.
Sulfadiazine and
sulfisoxazole still play a useful role in the prophylaxis of group A
streptococcal infections in patients with rheumatic fever who are
hypersensitive to penicillin. This is tempered with the potential for toxic-ity
and infection with resistant Streptococcus
pyogenes.
Trisulfapyrimidine (a
combination of sulfadiazine, sulfamerazine, and sulfamethazine), trimethoprim–
sulfamethoxazole, or sulfisoxazole can be used as an al-ternative drug for the
treatment of melioidosis caused by Pseudomonas
pseudomallei and for infections pro-duced by Nocardia spp.
A number of infections caused
by Chlamydia tra-chomatis, such as
trachoma, inclusion conjunctivitis, pneumonia,
and urethritis, can be treated with topical or systemic sulfonamides, although
tetracycline or erythro-mycin is preferred.
Sulfonamides, such as
sulfadiazine, in combination with pyrimethamine, are considered the treatment
of choice of symptomatic toxoplasmosis. Patients should be well hydrated to
prevent crystalluria; this problem may be reduced with the use of triple sulfas
(trisulfapyrimi-dine). Some regimens have included a sulfonamide (sul-fadoxine)
in combination with pyrimethamine (Fansidar)
for the treatment of chloroquine-resistant malaria caused by P. falciparum.
Topically active sulfonamides
are useful in prevent-ing infections in burn patients. Mafenide acetate(Sulfamylon Cream), the most widely used
compound, is effective against P. aeruginosa,
an organism that fre-quently colonizes burns. It is less effective against
staphylococci, which also colonize burns. Local absorp-tion of the acetate
preparation, which is acidic, can re-sult in respiratory alkalosis. Silver
sulfadiazine in a 1% cream can be used as an alternative to mafenide and has
good activity against gram-negative bacteria.
Sulfacetamide is used
topically for treatment of oc-ular infections.
Adverse Effects and Drug Interactions
If the concentration of the
sulfonamide is sufficiently high and its aqueous solubility is sufficiently
low, the free drug or its metabolites may form crystals and cause bleeding or
complete obstruction of the kidneys. Combinations of sulfa compounds have been
devel-oped for the purpose of lowering the dosage of individ-ual components to
reduce the chance of crystalluria (e.g., triple sulfas, such as the
trisulfapyrimidines).
The sulfonamides do cause
hypersensitivity reac-tions (e.g., rashes, eosinophilia, and drug fever) in a
small number of patients. Other rare allergic reactions include vasculitis,
photosensitivity, agranulocytosis, and thrombocytopenia. Stevens-Johnson
syndrome is also associated with sulfonamide use; it is characterized by fever,
malaise, erythema multiforme, and ulceration of the mucous membranes of the
mouth and genitalia. Hemolytic anemia may develop in persons with a ge-netic
deficiency of red blood cell glucose-6-phosphate dehydrogenase (G6PD).
Sulfonamides compete for
sites on plasma proteins that are responsible for the binding of bilirubin. As
a re-sult, less bilirubin is bound, and in the newborn, the un-bound bilirubin
can be deposited in the basal ganglia and subthalamic nuclei, causing kernicterus, a toxic en-cephalopathy.
For this reason, sulfonamides should not
be administered to newborns or to women
during the last 2 months of pregnancy.
Significant drug–drug
interactions are those that po-tentiate the effects of other agents and require
dosage modification. These include certain anticoagulants, hy-poglycemic sulfonylureas,
and hydantoin anticonvul-sants.
Related Topics