Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Radiochemical Methods of Analysis
Radiochemical Methods of Analysis
Atoms with the same number
of protons but a different number of neutrons
are called isotopes.
To identify an isotope we use the symbol A/zE, where E is the ele- ment’s atomic symbol, Z is
the element’s atomic
number (which is the number
of protons), and A is
the element’s atomic
mass number (which
is the sum of the number of protons and neutrons). Although
isotopes of a given element
have the same chemical
properties, their nuclear properties are different. The most impor- tant difference between isotopes is their stability. The nuclear configuration of a sta- ble isotope remains constant
with time. Unstable
isotopes, however, spontaneously disintegrate, emitting
radioactive particles as they transform into a more stable form.
|
2 |
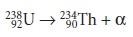
Beta particles, β, come in two forms.
A negatron, 0-1β is equivalent to an electron, and is produced when
a neutron is converted to a proton,
increasing the atomic number by 1.
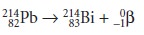
Converting a proton to a neutron results in the emission of a positron, .
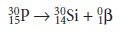
Emission of an alpha or beta particle often produces an isotope in an unstable, high-energy state.
This excess energy
is released as a gamma ray, γ, or an X-ray. Gamma ray and X-ray
emission may also occur without
the release of alpha or beta
particles.
Although similar to chemical kinetic
methods of analysis, radiochemical meth-
ods are best classified as nuclear kinetic
methods. In this section we review the ki-
netics of radioactive decay and examine several quantitative and characterization applications.
Related Topics