Chapter: Mechanical and Electrical : Thermal Engineering : Gas Power Cycles
Process of Otto cycle, Diesel cycle, Dual combustion cycle and Brayton cycle
CYCLES AND THEIR CONCEPTS
OTTO CYCLE
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines. The idealized diagrams of a four-stroke Otto cycle Both diagrams
Ø Petrol and gas engines are operated on this cycle
Ø Two reversible isentropic or adiabatic processes
Ø Two constant volume process
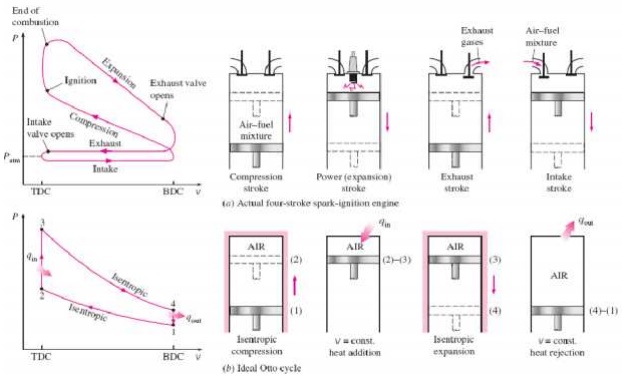
PROCESS OF OTTO CYCLE
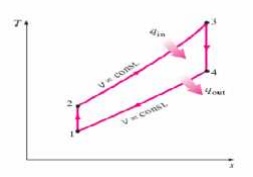
Ø Ideal Otto Cycle
Ø Four internally reversible processes
o 1-2 Isentropic compression
o 2-3 Constant-volume heat addition
o 3-4 Isentropic expansion
o 4-1 Constant-volume heat rejection
Thermal efficiency of ideal Otto cycle:
Since V2= V3 and V4 = V 1
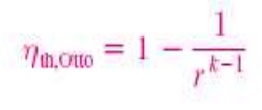
Diesel cycle
The Diesel cycle is a co mbustion process of a reciprocating internal co mbustion engine. In it, fuel is ignited by hea t generated during the compression of air in the combustion chamber, into which fuel is t hen injected.
It is assumed to have constant pressure during the initial part of the "combustion" phase The Diesel engine is a heat engine: it converts heat into work. During the bottom isentropic processes (blue), energy is transferred into the system in the form of work , Win but by definition (isentropic) no ene rgy is transferred into or out of the system in the form of heat. During the constant pressure ( red, isobaric) process, energy enters the syste m as heat . During the top isentropic processes (yellow), energy is transferred out of the sy stem in the form of Wout, but by definition (isen tropic) no energy is transferred into or out of the system in the form of heat. During the constan t volume (green,isochoric) process, some of energy flows out of the system as heat through the right depressurizing process Qout . The wor k that leaves the system is equal to the work that enters the system plus the difference between the heat added to the system and the heat that lea ves the system; in other words, net gain of wo rk is equal to the difference between the heat adde d to the system and the heat that leaves the system.
PROCESSES OF DIESEL CYCLE:
Ø 1-2 Isentropic com pression
Ø 2-3 Constant-Pres sure heat addition
Ø 3-4 Isentropic expansion
Ø 4-1 Constant-volume heat rejection
For ideal diesel cycle
Cut off ratio rc
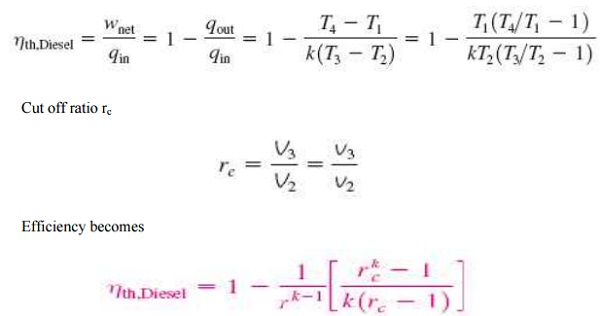
DUAL CYCLE
The dual combustion cycle (also known as the limited pressure or mixed cycle) is a thermal cycle that is a combination of the Otto cycle and the Diesel cycle. Heat is added partly at constant volume and partly at constant pressure, the advantage of which is that more time is available for the fuel to completely combust. Because of lagging characteristics of fuel this cycle is invariably used for diesel and hot spot ignition engines.
Ø Heat addition takes place at constant volume and constant pressure process .
Ø Combination of Otto and Diesel cycle.
Ø Mixed cycle or limited pressure cycle
PROCESS OF DUAL CYCLE

Ø Isentropic compression
Ø Constant-volume heat rejection
Ø Constant-pressure heat addition
Ø Isentropic expansion
Ø Constant-volume heat rejection
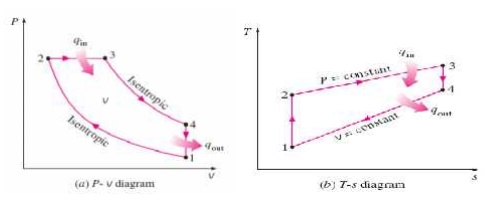
The cycle is the equivalent air cycle for reciprocating high speed compression ignition engines. The P-V and T-s diagrams are shown in Figs.6 and 7. In the cycle, compression and expansion processes are isentropic; heat addition is partly at constant volume and partly at constant pressure while heat rejection is at constant volume as in the case of the Otto and Diesel cycles.
BRAYTON CYCLE
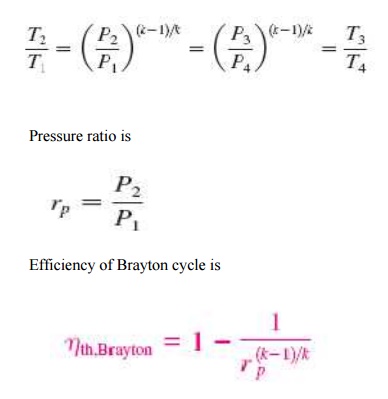
The Brayton cycle is a thermodynamic cycle that describes the workings of a constant pressure heat engine. Gas turbine engines and airbreathing jet engines use the Brayton Cycle. Although the Brayton cycle is usually run as an open system (and indeed must be run as such if internal combustion is used), it is conventionally assumed for the purposes of thermodynamic analysis that the exhaust gases are reused in the intake, enabling analysis as a closed system. The Ericsson cycle is similar to the Brayton cycle but uses external heat and incorporates the use of a regenerator.
Ø Gas turbine cycle
Ø Open vs closed system model
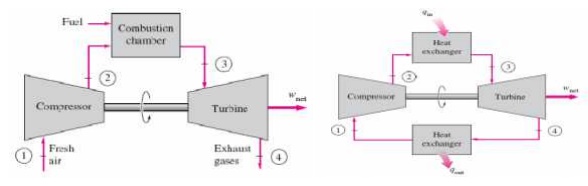
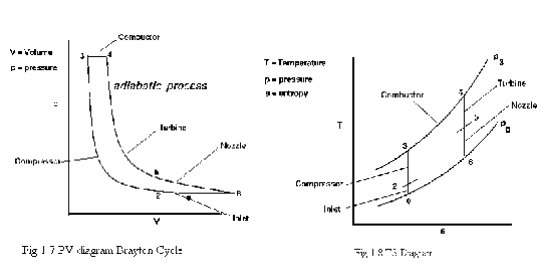
With cold-air-standard assumptions
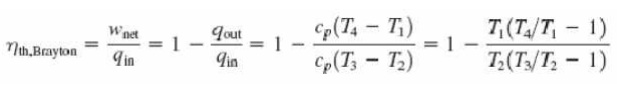
Ø Since processes 1-2 and 3-4 are isentropic, P2 = P3 and P4 = P1
Related Topics