Chapter: Medical Physiology: Energetics and Metabolic Rate
Phosphocreatine Functions as an Accessory Storage Depot for Energy and as an ŌĆ£ATP BufferŌĆØ
Phosphocreatine Functions as an Accessory Storage Depot for Energy and as an ŌĆ£ATP BufferŌĆØ
Despite the paramount importance of ATP as a coupling agent for energy transfer, this substance is not the most abundant store of high-energy phosphate bonds in the cells. Phosphocreatine, which also contains high-energy phosphate bonds, is three to eight times as abundant. Also, the high-energy bond (~) of phospho-creatine contains about 8500 calories per mole under standard conditions and as much as 13,000 calories per mole under conditions in the body (37┬░C and low con-centrations of the reactants). This is slightly greater than the 12,000 calories per mole in each of the two high-energy phosphate bonds of ATP. The formula for crea-tinine phosphate is the following:
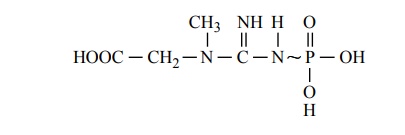
Unlike ATP, phosphocreatine cannot act as a direct coupling agent for energy transfer between the foods and the functional cellular systems, but it can transfer energy interchangeably with ATP. When extra amounts of ATP are available in the cell, much of its energy is used to synthesize phosphocreatine, thus building up this storehouse of energy. Then, when the ATP begins to be used up, the energy in the phosphocreatine is transferred rapidly back to ATP and then to the func-tional systems of the cells. This reversible interrelation between ATP and phosphocreatine is demonstrated by the following equation:

Note particularly that the higher energy level of the high-energy phosphate bond in phosphocreatine (1000 to 1500 calories per mole greater than that in ATP) causes the reaction between phosphocreatine and ADP to proceed rapidly toward the formation of new ATP every time even the slightest amount of ATP expends its energy elsewhere. Therefore, the slightest usage of ATP by the cells calls forth the energy from the phos-phocreatine to synthesize new ATP. This effect keeps the concentration of ATP at an almost constant high level as long as any phosphocreatine remains. For this reason, we can call the ATP-phosphocreatine system an ATP ŌĆ£bufferŌĆØ system. One can readily understand the importance of keeping the concentration of ATP nearly constant, because the rates of almost all the metabolic reactions in the body depend on this constancy.
Related Topics