Chapter: Medical Physiology: Energetics and Metabolic Rate
Control of Energy Release in the Cell
Control of Energy Release in the Cell
Rate Control of Enzyme-Catalyzed Reactions. Before dis-cussing the control of energy release in the cell, it is nec-essary to consider the basic principles of rate control of enzymatically catalyzed chemical reactions, which are the types of reactions that occur almost universally throughout the body.
The mechanism by which an enzyme catalyzes a chemical reaction is for the enzyme first to combine loosely with one of the substrates of the reaction. This alters the bonding forces on the substrate sufficiently so that it can react with other substances. Therefore, the rate of the overall chemical reaction is determined by both the concentration of the enzyme and the concen-tration of the substrate that binds with the enzyme. The basic equation expressing this concept is as follows:
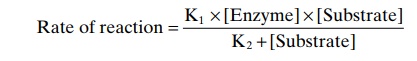
This is called the Michaelis-Menten equation. Figure 72–2 shows the application of this equation.
Role of Enzyme Concentration in Regulation of Metabolic Reactions. Figure 72–2 shows thatwhen the substrate ispresent in high concentration, as shown in the right halfof the figure, the rate of a chemical reaction is deter-mined almost entirely by the concentration of the enzyme. Thus, as the enzyme concentration increases from an arbitrary value of 1 up to 2, 4, or 8, the rate of the reaction increases proportionately, as demonstrated by the rising levels of the curves. As an example, when large quantities of glucose enter the renal tubules in a person with diabetes mellitus—that is, the substrate glucose is in great excess in the tubules—further increases in tubular glucose have little effect on glucose reabsorption, because the transport enzymes are satu-rated. Under these conditions, the rate of reabsorption of the glucose is limited by the concentration of the transport enzymes in the proximal tubular cells, not by the concentration of the glucose itself.
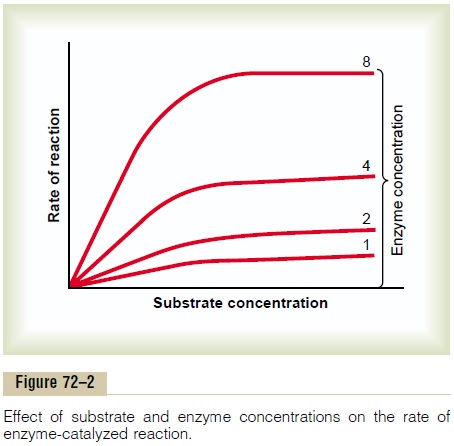
Role of Substrate Concentration in Regulation of Meta- bolic Reactions. Note also in Figure 72–2 that when thesubstrate concentration becomes low enough that only a small portion of the enzyme is required in the reac-tion, the rate of the reaction becomes directly propor-tional to the substrate concentration as well as the enzyme concentration. This is the relationship seen in the absorption of substances from the intestinal tract and renal tubules when their concentrations are low.
Rate Limitation in a Series of Reactions. Almost all chemicalreactions of the body occur in series, with the product of one reaction acting as a substrate for the next reac-tion, and so on. Therefore, the overall rate of a complex series of chemical reactions is determined mainly by the rate of reaction of the slowest step in the series. This is called the rate-limiting step in the entire series.
ADP Concentrationasa Rate-Controlling Factorin Energy Release. Underrestingconditions, the concentration ofADP in the cells is extremely slight, so that the chemi-cal reactions that depend on ADP as one of the sub-strates are quite slow. They include all the oxidative metabolic pathways that release energy from food, as well as essentially all other pathways for the release of energy in the body. Thus, ADP is a major rate-limitingfactor for almost all energy metabolism of the body.
When the cells become active, regardless of the type of activity, ATP is converted into ADP, increasing the concentration of ADP in direct proportion to the degree of activity of the cell. This ADP then automatically increases the rates of all the reactions for the metabolic release of energy from food. Thus, by this simple process, the amount of energy released in the cell is con-trolled by the degree of activity of the cell. In the absence of cellular activity, the release of energy stops because all the ADP soon becomes ATP.
Related Topics