Chapter: Paediatrics: Pharmacology and therapeutics
Paediatrics: Adverse drug reactions
One in 10 children in hospital and
one in 100 attending outpatients will experience an adverse drug reaction
(ADR). 71 in 8 will be severe. ADRs are responsible for almost 2% of children
admitted to hospital. ADRs in children can be as varied as in adults. Children,
because of growth and development, also suffer specific ADRs. Differences in
drug metabolism make certain ADRs a greater problem in children, e.g. valproate
hepato-toxicity, or less of a problem, e.g. paracetamol hepatotoxicity following
an overdose. The mechanisms of ADRs specifically affecting children are
illustrated with examples below.
Mechanisms of ADRs
Impaired drug metabolism
Chloramphenicol, when first used
in neonates, led to the development of the grey baby syndrome (vomiting,
cyanosis, cardiovascular collapse, and in some cases death). The newborn infant
metabolizes chloramphenicol more slowly than adults and so only requires a
lower dose of the antibi-otic. Reduction in the dosage prevents grey baby
syndrome.
Children, particularly neonates,
are more likely to have a reduced capac-ity to metabolize drugs than adults.
Therefore, lower doses are usually required.
Altered drug metabolism
Children may have reduced activity
of the major hepatic enzymes associ-ated with drug metabolism. To compensate
for this, they may use other enzyme pathways. This is thought to be one of the
factors contributing to the increased risk of hepatotoxicity in children under
the age of 3 years who receive sodium valproate. This risk is raised by the
concurrent use of other anticonvulsants which may cause enzyme induction of
certain metabolic pathways.
Sodium valproate should not be
used as a first-line anticonvulsant in children under the age of 3yrs.
Protein-displacing effect on bilirubin
The use of the sulphonamide
sulphisoxazole in ill neonates in the 1950s was associated with the development
of fatal kernicterus due to drug dis-placement of protein bound bilirubin into
the blood because of its higher binding affinity to albumin. Ceftriaxone also
is highly protein bound and will displace bilirubin in sick neonates.
The protein-displacing effect of
medicines should be considered in sick preterm neonates.
Percutaneous absorption
Percutaneous
toxicity can be a significant prob-lem in the neonatal period due to their
higher surface area to weight
ratio than that of both children
and adults. An example of this is the use of antiseptic agents such as
hexachlorophene that have been associated with neurotoxicity.
Drug interactions
Ceftriaxone
and calcium containing solutions when used
together in neonates may result in the precipitation of ceftriaxone— calcium
salt in the lungs. This drug interaction can result in fatalities and therefore
ceftriaxone should be avoided in neonates.
Ceftriaxone should be avoided in
neonates.
Unknown
There are several examples of
major ADRs that occur in children for which we do not understand the mechanism.
Salicylate given during the presence of a viral illness increases the risk of
the development of Reye’s syndrome in children of all ages. Since the use of
salicylates has been avoided in chil-dren the incidence of Reye’s syndrome has
dramatically reduced. Propofol has minimal toxicity when used to induce general
anaesthesia. Used as a sedative in critically ill children, however, it has
been associated with the death of over 10 children in the UK alone. The
propofol infusion syn-drome is thought to be related to the total dose of
propofol infused, i.e. high dose or prolonged duration is more likely to cause
problems.
Propofol should not be used as a
sedative in critically ill children
Suspect ADRs
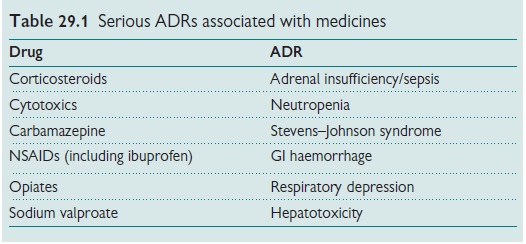
One should always
consider the possibility of an ADR being responsible for a child’s
symptoms. Table 29.1 lists some of the seri-ous ADRs associated with widely used
medicines.
Preventing ADRs
Recognizing which
patients are at greater risk of ADRs can help reduce the overall
incidence. Health professionals should follow guidelines.
Reporting ADRs
Suspected ADRs
should be reported to the regula-tory authorities. In the UK the yellow card
scheme is in operation.
Related Topics