Chapter: Modern Medical Toxicology: Cardiovascular Poisons: Anticoagulants and Related Drugs
Oral Anticoagulants - Cardiovascular Poison
Oral Anticoagulants
The commonest agent involved in overdose (or
rodenticide-related poisoning) is warfarin (coumafene). Brodifacoum,
dife-nacoum, and bromadiolone are 4-hydroxycoumarin derivatives with a 4-bromo
(1-1 biphenyl) side chain. Coumatetralyl is a 4-hydroxy coumarin derivative
rodenticide which most likely produces a long acting anticoagulant effect. It
differs from brodifacoum in having a 2H-1-benzopyran-2-one group in place of
the 4-bromo (1 -1 biphenyl) group found in brodifacoum. Chlorophacinone,
diphacinone and pindone are indandione anticoagulants with a long duration of
action. All these agents produce a more potent and persistent anticoagulant
effect than warfarin or other coumarin compounds.
Mode of Action
·
All oral anticoagulants act by
inhibiting vitamin K (which is a cofactor in the post-ribosomal synthesis of
clotting factors II, VII, IX, and X), by interfering with the activity of
vitamin K 2,3-epoxide reductase and vitamin K quinone reductase.
·
Platelet count, fibrinogen level,
and the concentrations of other clotting factors remain unaffected. Fibrin
split prod-ucts may be elevated
·
In overdose with long acting
anticoagulants, PT prolonga-tion and clinical bleeding have persisted for 45
days to 8 months.
Toxicokinetics
■■ Following oral
administration, warfarin is bioavailable to the extent of 100%, peaking in the
plasma in about 1 hour, with a volume of distribution of 0.126 L/kg and
protein-binding of 98 to 99%.
■■ It is metabolised by
oxidation to 6-hydroxywarfarin and 7-hydroxywarfarin (inactive), and by
reduction to diaste-reoisomeric alcohols.
■■ Elimination
half-life is about 40 hours. Duration of action may extend upto 5 days.
Adverse Effects
·
Haemorrhage, drop in haematocrit,
vomiting, diarrhoea, hepatic dysfunction, jaundice, pancreatitis, and cutaneous
reactions—skin eruptions (papular, vesicular, urticarial, or purpuric),
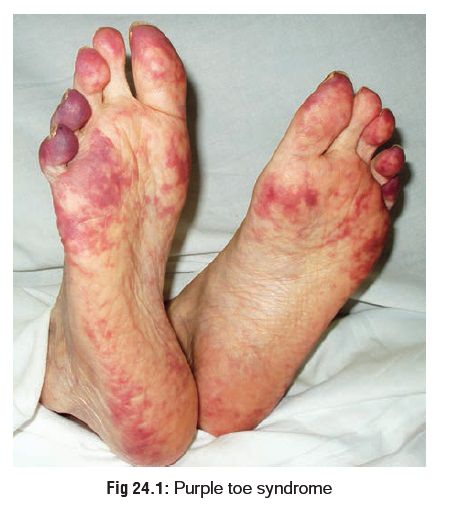
ecchymosis, purpura, purple toe syndrome
(Fig.24.1), and skin necrosis.
o Purple
toe syndrome is due to small atheroemboli which are no longer adherent to their
plaques by clot.
o Patients
with protein C, protein S, and antithrombin deficiencies are at increased risk
for skin necrosis. The common sites for necrosis include breasts, thighs, and
buttocks.
· The gastrointestinal tract is the site of bleeding in most of the patients. Upper airway bleeding may result in pain, dysphonia, dysphagia, dyspnoea and inability to clear secretions. Intracranial haemorrhage and haematomyelia may occur following warfarin therapy.
·
Haematomyelia, an uncommon
occurrence, has been reported following warfarin therapy. Symptoms include
paresis, back or neck pain, and urinary incontinence. ACT scan or magnetic
resonance imaging (MRI) usually confirms the diagnosis.
·
Hypotension occurs as a result of
hemorrhage due to warfarin therapy, particularly in patients who are over
anticoagulated.
·
Alopecia is reported to occur after
both acute and chronic use. The response is directly related to the highest
dose given and not to the duration of treatment. Hair is shed diffusely two or
three months after an adequate dose of the drug.
·
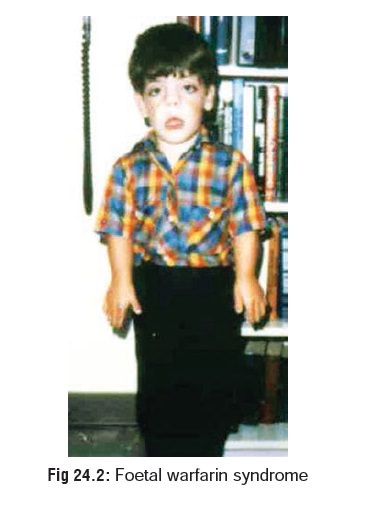
Warfarin or other coumarins, if
administered during preg- nancy (especially the first trimester) can cause a
malforma- tion syndrome—warfarin
embryopathy.
o Craniofacial,
musculoskeletal, skin, eye, gastrointes-tinal, and cardiovascular developmental
abnormali- ties have been observed in the offspring of women administered
warfarin during pregnancy (Fig 24.2).
It causes characteristic skeletal anomalies when given in the first trimester,
and central nervous system defects when given later in pregnancy.
o When
warfarin is given during the first trimester, nasal hypoplasia, respiratory
deficiency secondary to nasal obstruction, dextrocardia, abdominal situs
inversus, retardation, calcified stippling of secondary epiphyses, reduced birth
weight, rhizomelia (short proximal limbs), scoliosis, and short phalanges have
been reported.
o In
addition to teratogenicity due to first trimester exposure, second and third
trimester exposure has been associated with microcephaly, retardation, and optic
atrophy. A variety of ophthalmic disorders have been reported, including optic
atrophy, large eyes, microphthalmos, and opacified lenses.
o Heparin
does not cross the placental barrier and therefore can be given during
pregnancy.
Drug Interactions
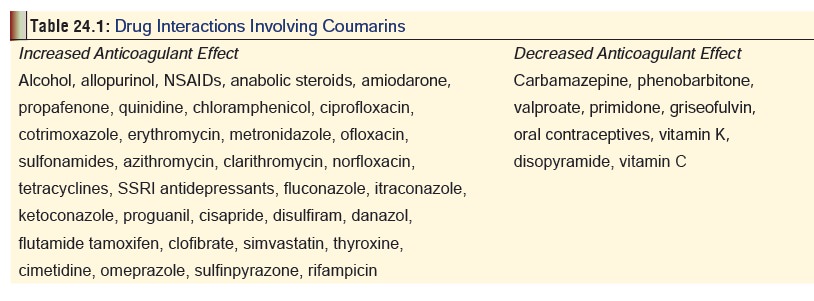
Table 24.1
represents a summary of important drug interactionsinvolving
coumarins.
Toxic (Clinical) Features
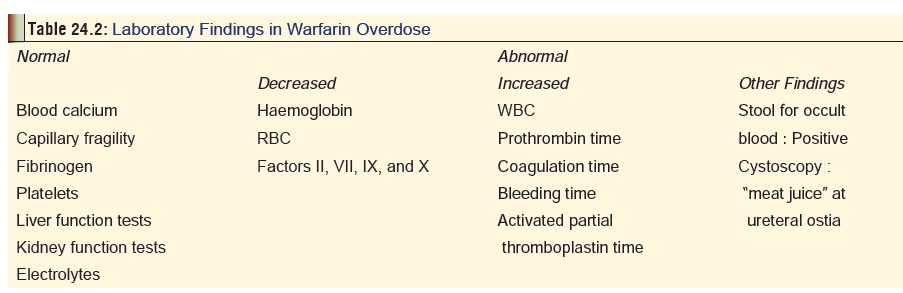
· Overdose with coumarins leads to
bleeding in multiple organ sites that can prove life-threatening. In massive
overdose, these agents have produced rapid and persistent hypopro-thrombinaemia
and associated bleeding diathesis. Table24.2
lists some of the laboratory (and other investigative)findings.
· Warfarin may lead to toxic effects
by ingestion, inhalation, and intravenous administration. It is moderately toxic
by dermal, subcutaneous, and intraperitoneal routes.
· The primary effect of warfarin
overdose is prolongation of prothrombin time, and subsequent risk of
haemorrhage.
·
The onset of prolonged PT correlates with the half-life of
factor VII, usually appears within 24 hours of ingestion, and peaks between 36
to 72 hours. Clinical manifestations begin a few days or weeks after ingestion,
and include epistaxis, gingival bleeding, pallor, haematuria, haematochezia,
melaena, and haematomas around joints and on buttocks. Other symptoms include
back pain, bleeding lips, mucous membrane haemorrhage, abdominal pain,
vomiting, and petechial rash. Later, paralysis due to cerebral haemorrhage, and
finally haemorrhagic shock and death may occur.![]()
· Long-acting anticoagulants are about
100 times more potent than warfarin on a mole for mole basis. In addition, they
have a much longer duration of action which can sometimes last for weeks or
months. While the onset of prolonged prothrombin times occurs generally within
48 hours, the first clinical signs of bleeding may be delayed until one to four
weeks after ingestion. Common manifestations in such cases include purpura, GI
bleeding, haematemesis, haemoptysis, epistaxis, haematuria, melaena,
menorrhagia, and CNS bleeds. Multiple ecchymoses and haematomas may be evident
on physical examination. Chest pain and tachycardia may develop secondary to
blood loss.
Treatment
Investigations:
·
Plasma levels of warfarin can be measured by a variety of
techniques, but are not generally obtained to monitor the clinical course in
poisoning cases.
·
The international normalised ratio (INR) or prothrombin time
(PT) are the best values to monitor. The onset of INR elevation or PT
prolongation is between 12 and 24 hours post-ingestion. Any increase in INR or
prolongation of prothrombin time when compared to normal controls, indicates
toxicity. The risk of bleeding is minimal with a PT of 1.3 to 1.5 times
control. At PT of 2 times control or greater there is an exponentially
increased risk of bleeding. In the case of long acting anticoagulants, INR or
prothrombin times may be normal 24 hours post-ingestion, and become prolonged
at 48 hours or later, therefore 24 and 48 hour PT (or INR) has been
recommended.
·
Determination of blood clotting factors II, VII, IX, and X
may be helpful in guiding therapy in symptomatic patients. Since clotting
factors may be abnormal with a normal INR or PT, they are a more sensitive
measure of toxicity and may be more useful in guiding vitamin K1
therapy.
·
Monitor haemoglobin and haematocrit if bleeding occurs.
Monitor urine and stool for occult blood. Various imaging studies may be
helpful in diagnosing spontaneous haem-orrhage into various tissues or body
compartments.
Stabilisation:
·
Admit to intensive care facility and monitor clotting
parameters. Watch out for signs of bleeding or bruising. Coagulopathy may
persist for 6 weeks or longer in patients who ingest large amounts of long
acting anti-coagulants in suicidal attempts. Premature discharge of such
patients at 3 to 4 weeks postingestion prior to full normalisation of factor
levels has resulted in fatalities.
·
Frequent outpatient monitoring should be done on patients
discharged on oral vitamin K1 to ensure compli-ance and adequacy of
treatment. Factor assays should be normal prior to discontinuation of vitamin K1.
·
Administer whole blood or plasma if bleeding is severe.
Decontamination:
·
Emesis and gastric lavage are
contraindicated due to the potential risk of inducing bleeding.
·
Activated charcoal can be
administered. Patients on chronic anticoagulation therapy should receive
acti-vated charcoal after an acute overdose unless contrain-dicated.
Antidote:
Vitamin K1(phytomenadione,
phytonadione,phylloquinone).
·
Mode of action: Since oral
anticoagulants are vitamin K antagonists, administration of vitamin K1
sets right the anomaly. Vitamins K2
(menaquinones), K3
(menadione), and K4 (menadiol
sodium diphosphate) are not recom-mended, since they can induce haemolysis,
hyperbiliru-binaemia, and kernicterus in neonates, and haemolysis in G6PD
deficient patients.
·
Indications: Prophylactic treatment
for a suspected large ingestion of warfarin is not recommended. PT or INR
should be checked 24 hours after ingestion. If results are normal, PT and INR
should be repeated at 48 hours after ingestion. If PT or INR is elevated, then
Vitamin K1 may be given.
Dose:
––
Oral—50 to 100 mg, 3 to 4 times a day, for 1 to 2 days, (adults); 10 to 25
mg/day, (children).
––
Subcutaneous—25 to 50 mg, 2 to 4 times a day. –– Intravenous—25 to 50 mg
(diluted in normal saline or glucose), given slowly, 2 to 4 times a day. Rapid
IV administration can cause facial flushing, sweating, chest pain, hypotension,
dyspnoea.
––
Intramuscular use can result in haematoma forma-tion.
––
Anaphylactoid reactions have been reported with vitamin K1.
Supportive measures:
·
Administer fresh frozen plasma
and/or prothrombin complex concentrate and packed red blood cells as needed for
significant active bleeding. The usual dose of fresh frozen plasma given to
correct coagulation factor deficiency is 15 ml/kg, but the recommended dose
required to reverse over anticoagulation due to warfarin has not been established.
·
Since long-acting anticoagulants are
metabolised by the hepatic mixed-function oxidase system (cytochrome P450),
phenobarbitone 100 to 200 mg/day, may be helpful in reducing the duration of
coagulopathy by inducing the hepatic microsomal metabolism of these compounds.
Forensic Issues (Anticoagulants)
·
Anticoagulant poisoning may result
from accidental, suicidal or homicidal causes.
·
Accidental incidents are mostly the
result of therapeutic errors. Occasionally, childhood poisoning may result from
inadvertent consumption of products containing these agents, especially rat
poisons.
·
Suicidal intake of such rodenticides
is also quite commonly reported in India.
·
Covert use of long acting
anticoagulants may be a manifes-tation of child abuse or Munchausen syndrome.
Related Topics