Chapter: Surgical Pathology Dissection : Laboratory Techniques
Laboratory Techniques: Fixation
Fixation
Adequate
fixation by an appropriate fixative is central to any histologic preparation.
Tissue that is inadequately or inappropriately fixed will lead to difficulties
in microtomy, staining, and per-forming ancillary tests. These problems may not
be correctable at a later stage.
Unfortunately,
there is no ‘‘all-purpose’’ fixa-tive. No single fixative is good for all
specimens. It is therefore essential that surgical pathology personnel be
familiar with a variety of fixatives and their uses. Although the exact
mechanism of action of many fixatives is unknown, fixatives can broadly be
classified into four groups based on their mechanism of action. The aldehydes,
such as formaldehyde and glutaraldehyde, act by cross-linking proteins,
particularly lysine resi-dues. Oxidizing agents, such as osmium tetrox-ide,
potassium permanganate, and potassium dichromate, also probably cross-link
proteins, al-though their precise mechanism of action is un-known. Acetic acid,
methyl alcohol, and ethyl alcohol are all protein-denaturing agents. The fourth
and final group of fixatives acts by forming insoluble metallic precipitates,
and these agents include mercuric chloride and picric acid. The choice of the
appropriate fixative is based on the type of tissue being fixed and on
projected needs for ancillary tests such as special stains,
immunohistochemistry, in situ
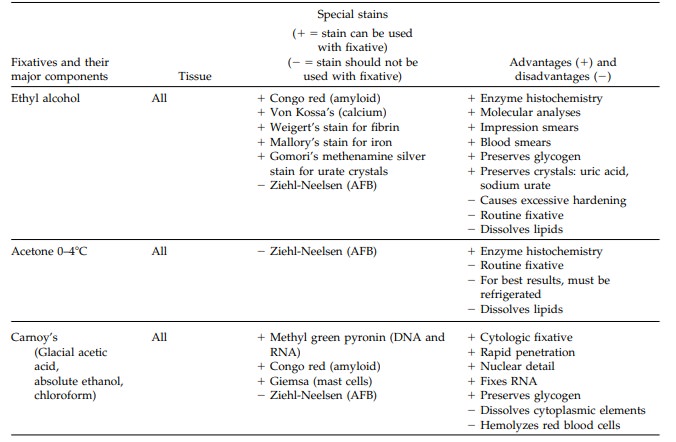
hybridization, and electron microscopy. Table 2-1 lists some common fixatives,
their basic uses, and their advantages and disadvantages.
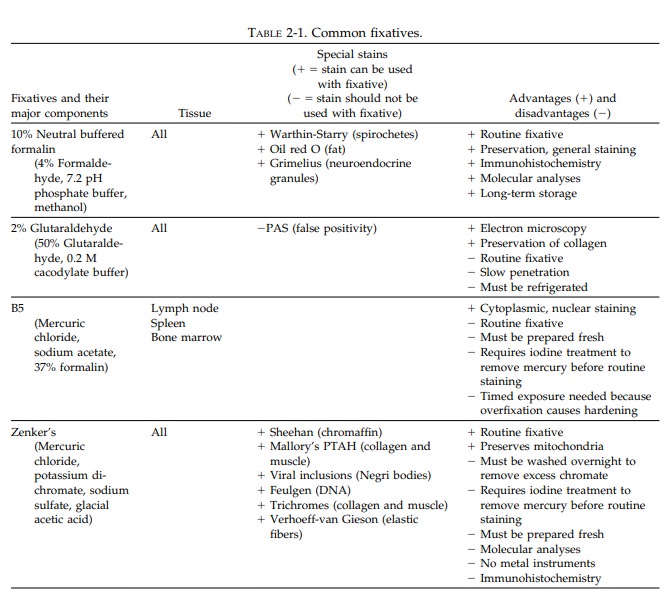

Ten
percent neutral buffered formalin (4% formaldehyde) is the standard fixative
used in most laboratories. Formalin tends to remove water-soluble substances
such as glycogen, and it is therefore generally not suitable for the fixa-tion
of tissues for electron microscopy. Ten per-cent neutral buffered formalin
penetrates and fixes tissues at a rate of approximately 2 to 3 mm/24 h at room
temperature.
Glutaraldehyde,
a common fixative for electron microscopy, is one of the slowest penetrating
fixatives. Tissue for electron microscopy should be cut into 1-mm cubes and immediately
placed in refrigerated glutaraldehyde. Glutaraldehyde (4%) must be kept
refrigerated before use.
Ethyl alcohol (70% to 100%) is seldom used as a primary fixative. It may be useful in fixing tissue for preserving glycogen and for some histochemi-cal studies, but it has several disadvantages. Ethyl alcohol penetrates tissues very slowly, and because it denatures proteins by abstracting water from the tissue, it can cause excessive hardening, tissue shrinkage, and cell distortion. Alcohol can also dissolve fats and should not be used when lipid studies or stains for myelin are being considered. Carnoy’s is a fixative that combines ethanol, chloroform, and glacial acetic acid. It quickly fixes tissues and it is a good fixative for glycogen, plasma cells, and nucleic acids. Because of its quick action, some labora-tories use Carnoy’s to fix biopsies that require urgent processing.
The
mercury-based fixatives (e.g., B5) provide excellent nuclear detail and are
useful in evaluat-ing lymphomas. Mercury-based fixatives precipi-tate proteins
without firmly binding to them. These fixatives generally must be prepared
fresh; once fixed, the tissues require special processing in the histology
laboratory (iodine treatment to remove the mercury). Overfixation with B5 can
cause excessive hardening of the tissue.
Bouin’s,
a picric acid-based fixative, is the fixa-tive of choice for testicular
biopsies. Picric acid reacts with basic proteins and forms crystalline picrates
with amino acids. Therefore, tissues fixed with picric acid-based fixatives
retain little affin-ity for basic dyes, and the picric acid must be recovered
from the tissue before staining. Picric acid penetrates tissues well and fixes
them rap-idly, but it also causes cells to shrink. Picric acid causes DNA
methylation; hence, many poly-merase chain reaction (PCR)-based molecular
diagnostic tests cannot be performed on tissues fixed with picric acid.
An
appropriate fixation technique is just as im-portant as choosing the correct
fixative. Appro-priate fixation requires adequate tissue exposure and a
duration of fixation sufficient to allow full penetration of the fixative. For
most tissues, a volume of fresh fixative 15 times the volume of tissue is
needed to fix the tissue adequately within 12 to 18 hours. The rate of fixation
var-ies depending on the type of fixative, the type of tissue, and the
thickness of the tissue sections. Adipose tissue (due to its hydrophobic
nature) and fibrous tissue (due to its density) may require longer periods of
fixation when hydrophilic fixa-tives are employed.
There
can be no more important tenet of fixa-tion than to do it early. The process of
autolysis begins immediately, and even the best fixative can only arrest, not
reverse, this process. Small amounts of tissue may arrive in fixative or
saline, whereas larger tissues usually arrive fresh. Large specimens generally
do not fix well un-less first prepared. Even then, specimens often require a
limited dissection to maximize the surface area exposed to the fixative,
thereby en-suring adequate fixation. Tissue with a hollow viscous or lumina
should be opened and solid tissue partially serially sectioned at 5- to 10-mm
intervals. To maintain proper orientation, these partially sectioned tissues
can be pinned onto a wax block and floated in a fixation tank. Paper towels can
be inserted between the sections. The towels act as a wick, drawing more
fixative to the sections, thereby facilitating rapid fixation. In general,
tissue submitted for processing should never exceed a thickness of 4 mm, and
tissues comprised of adipose or dense fibrous tissue should be no more than 3
mm. Optimally, you should routinely aim to submit your tissue sec-tions as 2 mm
slices. There should be at least a 3-mm space between the cassette and tissue
on all sides. Cramming oversized tissue to make it fit into a cassette often
results in inferior slide preparation, time consuming reprocessing, and
ultimately a delay in diagnosis.
Related Topics