Chapter: Modern Medical Toxicology: General Principles: Medicolegal Aspects of Poisoning
Indian Statutes on Drugs/Poisons
INDIAN STATUTES ON DRUGS/POISONS
Several
legal acts have been passed regulating and controlling the manufacture, sale,
distribution, and possession of drugs and poisons. The principal acts include
the following:
The Poisons Act (1919)
This
Act was amended in 1958 and repealed in 1960. It deals with the import of
poisonous substances into India, license issu-ance for possession of certain
specified poisons, and restrictions
Drugs And Cosmetics Act (1940)
This
Act was amended in 1964, and very recently in 2008, and is today referred to as
the Drugs And Cosmetics AmendmentAct
(2008). It deals with the import, manufacture, distribution,and sale of all
kinds of drugs (allopathic, ayurvedic, unani, siddha, etc.) and cosmetics. As
per the Act, every patented or proprietary medicinal preparation should display
on the label of the container, either the exact formula or a list of the
ingre-dients. The amended Act has enhanced the scale of punishment for various
offences, including sale of spurious drugs, adultera-tion of drugs and
cosmetics, toxic contamination, etc.
The Drugs And Cosmetics Rules (1945)
This
is an offshoot of the Drugs and
Cosmetics Act of 1940, and is
concerned mainly with the standard and quality of drugs, apart from exercising
control over the manufacture, sale, and distribution, of drugs and cosmetics.*
It was amended in 1988, and is now referred to as Drugs And Cosmetics Rules (Eigth Amendment) 1988. All types of
drugs used in therapeutics have been included: allopathic, homeopathic, ayurvedic, unani, and siddha. All drugs and cosmetics are required to be labelled and
packed appropriately. To advise the Central and State Governments on technical
matters relating Drugs Technical
Advisory Board, the Ayurvedic and Unani Technical Advisory Boards, and
the Drugs Consultative Committee.
In
order to facilitate the analysis or testing of drug samples to assess their
quality, the Central Drugs Laboratory
was established in 1962. Individual states have set up DrugControl Laboratories. Stringent punishments have been laid down for manufacture, stocking, or sale
of substandard or spurious drugs. Guidelines for conducting clinical trials for
new drugs have been made more strict (Schedule Y).
The Drugs and Cosmetics Rules have
classified drugs into various Schedules
as follows:
·
Schedule C and C1—Biological
products, e.g. serums and vaccines.
·
Schedule D—Substances not intended
for medicinal use—condensed or powdered milk, oats, spices and condiments, etc.
·
Schedule E1—List of poisonous
substances under the Ayurvedic (including Siddha) and Unani Systems of
Medicine.
·
Schedule G—Chemotherapeutic agents
for cancer, antihis-taminics, and hypoglycaemic agents.
·
Schedule H and L—Injectables,
antibiotics, antibacterials and other prescription drugs.
·
Schedule J—Diseases and ailments (by
whatever name described) which a drug may not purport to prevent or cure or
make claims to prevent or cure, e.g. AIDS, cancer, cataract, congenital
malformations, deafness, blindness, hydrocoele, hernia, piles, leucoderma,
stammering, paralysis, etc.
·
Schedule O—Standards to be followed
with regard to disinfectant fluids.![]()
·
Schedule S—Standards to be followed
with regard to cosmetics and allied products.
·
Schedule X drugs—Barbiturates and
certain other seda-tives, amphetamines, etc.
·
A list of drugs banned for sale in
India as per The Drugsand Cosmetics
Rules is listed in Appendix 2
The Pharmacy Act (1948)
The
objective of this Act is to allow only registered pharmacists to compound,
prepare, mix, or dispense any medicine on the prescription of a registered
medical practitioner. Under this Act, the Pharmacy
Council of India has been constituted which regulates the study of pharmacy
throughout the country. Individual states have State Pharmacy Councils for registra-tion of pharmacists.
The Drugs Control Act (1950)
This
Act regulates the supply and distribution of drugs, and also guides the
manufacturer or dealer in fixing the maximum price for every drug.
The Drugs and Magic Remedies (Objectionable Advertisement) Act (1954)
The
objective of this Act is to ensure that ethical standards are maintained when
drugs are advertised by the manufac-turers. Advertisements which offend decency
or morality can be banned under this Act. Also, those which claim magical
powers for certain drugs, e.g. enhancement of potency, cure for incurable
diseases, etc. Magical remedies include the use of talismans or charms such as
“mantras”, “kavachas”, etc.
The Medicinal And Toilet Preparation (Excise Duty) Act And Rules
This
Act deals with regulatory problems arising out of the use of alcohol in various
medicinal and toilet preparations. It has helped greatly in curbing the large
scale inter-state smuggling of alcoholic medicinal, and toilet preparations
which existed previously due to different rates of excise duties in different
states. This Act has made uniform rates of duty applicable throughout the
country.
Narcotic Drugs and Psychotropic Substances Act (1985)
The Narcotic Drugs and Psychotropic Substances Act(NDPS Act) was
enacted in India and subsequently amendedin 1988, to implement the provisions
of the Convention onPsychotropic
Substances (1971), and the Convention
Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances (1988),
both held in Vienna. This Act renders obsolete three ![]() previous Acts—the Opium
Act (1857), the Opium Act (1878),
and the Dangerous Drugs Act (1930).
previous Acts—the Opium
Act (1857), the Opium Act (1878),
and the Dangerous Drugs Act (1930).
The
term “narcotic” in the legal sense is quite different from that used in the
medical context which denotes a sleep-inducing agent. Legally, a narcotic drug
could be an opiate (a true narcotic), cannabis (a non -narcotic), or cocaine
(the very antithesis of a narcotic, since it is a stimulant). The term
“psychotropic substance” is with reference to mind-altering drugs such as LSD,
phencyclidine, amphetamines, barbiturates, methaqualone, benzodiazepines,
mescaline, psilocybine, and designer drugs (MDMA, DMT, etc.).
The
NDPS Act imposes complete prohibition
on the cultivation of coca, poppy, and cannabis plants, and the manu-facture,
sale, purchase, use, or transport of any narcotic drug or psychotropic
substance except for medical or scientific purposes.
The
minimum punishment for any offence committed under the Act is 10 years rigorous
imprisonment and fine of Rs.1 lakh, while the maximum punishment is 20 years
rigorous imprisonment and fine of Rs.2 lakhs. There is also sufficient scope
under the NDPS Act for enhanced
punishment for repeat offences especially after previous convictions, which
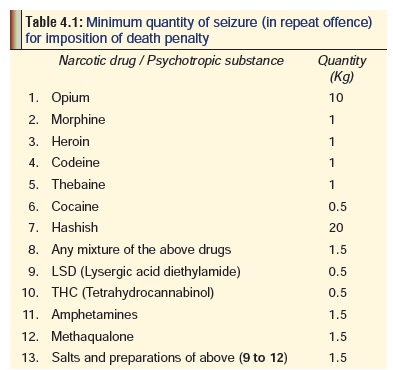
includes the imposition of even the death penalty. The minimum quan-tity of
drug seized in the subsequent offence should be as per Table 4.1, if death penalty is to be imposed. To constitute
anoffence the first time around, the minimum quantity seized should be equal to
or over 250 mg for heroin, 5 gm for hashish or charas, 5 gm for opium, 125 mg
for cocaine, and 500 gm for ganja.
In
addition to persons directly involved in trafficking narcotic drugs and
psychotropic substances, any person who finances trafficking, or harbours a
person involved in traf-ficking, or abets, or is a party to a criminal
conspiracy, including a criminal conspiracy to commit an offence outside India
is also liable to the same scale of punishments.
However,
immunity from prosecution is given to addicts volunteering for detoxification. Section 64 -A of the NDPSAct states that any addict who is
not charged with any offencepunishable under Sections 15 to 25,* or Section
27-A ,** and who voluntarily seeks to undergo medical treatment for
detoxification or de-addiction from a hospital or an institu-tion maintained or
recognised by the government or a local authority, and undergoes such treatment
shall not be liable to prosecution under Section
27 of the Act (once in his lifetime). Such immunity granted may be
withdrawn if the addict does not undergo the complete treatment, and in such
circumstances the accused shall be prosecuted for the said offence.
Further,
the Act makes a distinction between possession for personal consumption and
trafficking, the punishment for the former being limited to between six months
and one year only. The application of this provision is subject to the
following two qualifications:
The
quantity of the drug involved in the offence should be a small quantity as
specified by the Central Government.
The
onus is on the accused to establish that the drug in question was meant for
personal consumption and not for sale, distribution, etc.
The
Central Government of India constituted a NarcoticsControl
Bureau in 1986 with its headquarters at NewDelhi, and zonal offices at
Mumbai, Kolkata, Chennai, and Varanasi. In 1988, the Central Government
constituted the
Narcotic Drugs and Psychotropic Substances Consultative
Committee,
consisting of a chairman (the minister of finance/minister of state in the
ministry of finance), and 18 members from diverse fields who would among other
functions, conduct periodic review of the NDPS
Act.
While the NDPS Act prohibits the cultivation of poppy, cannabis, and coca
plants, it does not impose a total ban. Restricted cultivation of these plants
is allowed under strict control for scientific or medical use. Prior sanction
in the form of license is necessary from the Central Government for this purpose.
For instance, poppy can be cultivated only in certain specified tracts in the
states of Rajasthan, Uttar Pradesh,
and Madhya Pradesh during a specified
period, the opium yearcommencing on the first day of October every year, and
ending on the 30th day of September the following year. These policy controls
are backed by strict enforcement on the ground which include the measurement of
fields, periodical crop surveys, and physical checks to prevent diversion.
Failure to tender the entire yield to the Government
is treated as a serious offence and any cultivator who embezzles the opium
produced by him, is in terms of section 19 of the Act, punishable with rigorous
imprisonment for a term of between 10 to 20 years, and a fine which shall not
be less than Rs.100,000/- but which may extend to Rs.200,000/-.
The 1998 UN Convention against
Illicit Traffic in Narcotic Drugs and Psychotropic Substances to which India is
a signa-tory, requires countries to impose controls over the manufac-ture,
internal distribution, and import and export of chemicals which can be used in
the illicit manufacture of substances of abuse. In order to implement India’s
obligations under this Convention, the NDPS Act was amended in 1998 in order to
empower the Central Government to declare any substance as a controlled
substance and to regulate its manufacture, import and export, etc.. Violations
relating to such substances were established as criminal offences punishable
with imprisonment for upto 10 years. In 1993, the Government of India promulgated
the NDPS (Regulation of Controlled Substances) Order, to regulate the
manufacture, distribution, etc. of any substance declared to be a “Controlled
Substance”.
In exercise of its powers under the
Act, the Central Government has so far notified acetic anhydride, which is used
in the processing of opium into heroin, N-acetylanthranilic acid which is used
in the illicit manufacture of methaqualone, and ephedrine and pseudoephedrine
which are used in the illicit manufacture of amphetamine type stimulants as
“controlled substances”.
Related Topics