Chapter: Chemistry : Fuels and Combustion
Important Questions and Answers: Fuels and Combustion
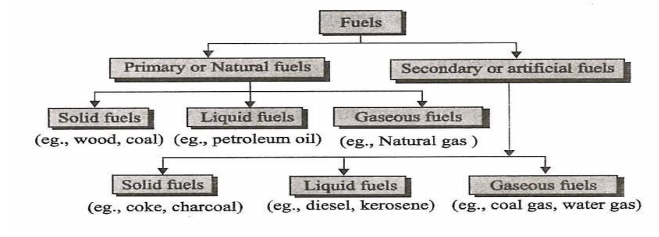
1. How fuels are classified. Give
one example for each.
Based on
Occurrence - 2 Types.
Primary Fuels – It occurs in nature such as. Ex:
Coal, Crude oil, Natural Gas.
Secondary
Fuels – It is derived from primary fuels. Ex: Coke, Petrol, Coal gas. Based on
physical state – 3 types
Solid fuels, eg., coal, coke,
Liquid fuels eg., gasoline, diesel.
Gaseous
fuels eg., coal gas, natural gas.
2. Define calorific value.
Explain higher & lower calorific value.
It is defined as the amount of heat obtainable by
the complete combustion of a unit mass of the fuel. Units of Calorific value ; Calorie,
kilocalorie, British thermal unit, centigrade heat unit Higher calorific value
/ Gross calorific value (GCV)
GCV = The
total amount of heat is produced, when a unit quantity of the fuel is
completely burnt and the products of combustion are cooled to room temperature
is known as GCV.
Lower
calorific value / Net calorific value (GCV)
NCV = The
total amount of heat is produced, when a unit quantity of the fuel is
completely burnt and products of combustion are allowed to escape is known as
NCV
NCV = GCV
-0.09H x 587.
3. Explain proximate analysis.
Give its significance.
It involves the determination of % of moisture
content, volatile matter, ash content & fixed carbon in coal.
(i) Moisture content: About 1
gram of air-dried powdered coal sample is taken in a crucible & it is
heated at 100-105°C for 1 hour.

(ii) Volatile matter: After
the analysis of moisture content, the crucible with residual coal sample is
converted with lid & it is heated upto 950°C for 7 minutes.
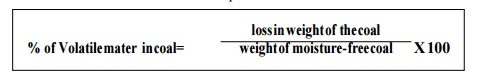
(iv) Fixed carbon: It is
determined by the subtracting the sum total of moisture, volatile & ash
content from 100.
% of fixed carbon in coal = 100 - % of (moisture
content + volatile matter + ash content)
Significance or importance of
Proximate Analysis:
High % of moisture is undesirable because it
reduces the calorific value of a fuel, & increases the transport cost.
High % of matter is undesirable because it reduces
the calorific value of a fuel & coal burns with a long flame & high
smoke.
High % of ash content is undesirable because it
reduces the calorific value of a fuel & makes the additional cost of
disposal of ash.
High % of fixed carbon in a coal, is greater its
calorific value.
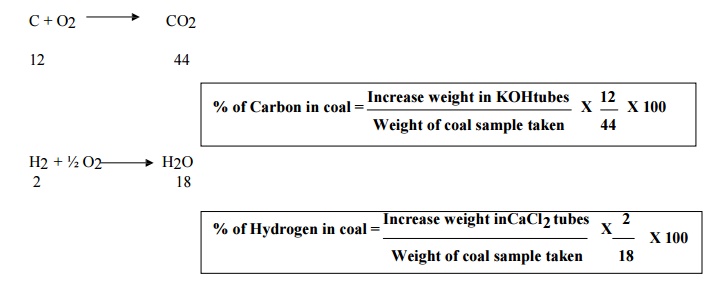
4. Explain ultimate analysis.
Give its significance.
It involves
the determination of % of Carbon, Hydrogen, Nitrogen, Sulphur & Oxygen in
coal.
1. Carbon & Hydrogen: A known
aount of coal sample is burnt with o2 in a combustion apparatus. In coal carbon & hydrogen are converted
into CO2 & H2O & those are absorbed by KOH tubes & CaCl2 tubes.
2. Nitrogen:
It is
carried out by Kjeldahl‟s method. A known amount of coal is heated with Con.
H2SO4 in presence of K2 SO4 catalyst in a long necked flask called Kjeldahl‟s
flask. Nitrogen is converted into ammonium sulphate. Then it is heated with
NaOH & absorbed by HCl.
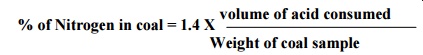
3. Sulphur: A known
amount of coal is completely burnt in a bomb calorimeter. Here sulphur is converted into sulphate
& treated with BaCl2, BaSO4 is obtained.
Oxygen: The % of
oxygen is calculated as follows,
of Oxygen
in Coal = 100 - % of ( C + H+N+S+ash)
Importance or significance of
Ultimate analysis:
Higher % of carbon & hydrogen, better is the
quality of coal & higher its calorific value.
Should have very little nitrogen content.
Presence of sulphur is undesirable because it forms
harmful gases during the combustion.
Lower of the Oxygen increases the higher its
calorific value.
How
Metallurgical coke is manufactured by Otto-Hoffman’s method?
When bituminous coal is heated strongly in the
absence of air, the volatile matter escapes out & the mass becomes hard,
strong, porous, which is called metallurgical
coke.
Manufacture of Metallurgical coke
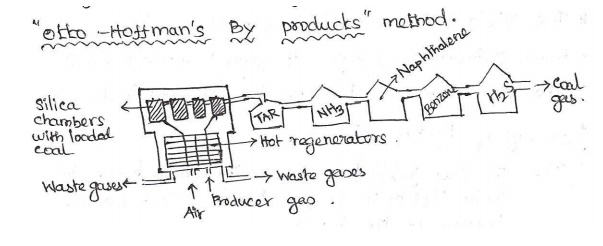
by “Otto- Hoffman’s by products” method:
In order to (i) Increase the thermal efficiency of
the carbonization process & (ii) Recover the valuable by products by this
method.
This oven
consists of a number of silica chambers.
Each
chamber is about 10-12 m long, 3-4 m height & 0.4 – 0.45m wide.
Coal is
introduced into the silica chambers & heated 1200°C by air & producer
gas. I & IV regenerators are heated by hot flue gases & II & III
regenerators are
heated by
incoming air & producergas.
When the
process is complete, the coke is removed & cool by with water. Time taken
for this process is 12-20 hours.
The yield
of the coke is about 70%
From out
coming flue gas, it gives valuable products like Tar, ammonia, benzene. H2S are
obtained.
Recovery of by products:
(i)
Tar: the flue gases are first passed through a
tower, in which liq. NH3 is sprayed, tar is collected at the bottom of the
tank.
(ii)
Ammonia: The gases are then passed through 2nd
tower, in which water is sprayed & NH3 gets in the form of NH4OH.
(iii)
Naphthalene: The gases are again passed through
next tower, cooled water is sprayed, Naphthalene gets & condensed.
(iv) Benzene: The gases are passed through next
tower, petroleum oil is sprayed, benzene gets condensed.
(v) H2S gas: The remaining gases are then passed
through a purifier, H2S gas is obtained. The final gas left out is called coal
gas.
Advantages:
(i)
Valuable by product like NH3, benzene, etc are obtained.
The carbonization time is less.(iii) Heating is
done by extremely by producer gas.
What do you mean by hydrogenation of coal? How
Synthetic petrol is manufactured by Bergius Process? Or How solid fuel is converted
into liquid fuel? Explain in detail.
The
preparation of liquid fuels from solid coal is known as hydrogenation of coal
& this gasoline is known as synthetic petrol.
Bergius
Process – Direct method:
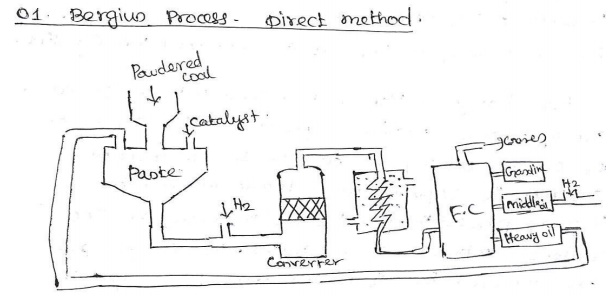
Input :
Powdered coal + Ni Oleate catalyst + Heavy oil are made into paste + H
Heating
in : 400 - 450°C.
Pressure
: 200-250atm.
Process: Powdered coal is converted into gasoline.
In this
process powdered coal is mixed with Ni oleate & heavy oil & made into
paste.
It is
pumped along with H2 gas into the converter, the paste is heated to 400 - 450°C
under pressure of 200-250atm.
Crude oil
comes & it is fractionated into 3 parts.
1.
Gasoline. 2. Middle oil. 3. Heavy oil.
The
middle oil is further hydrogenated in vapour phase to get gasoline. The heavy
oil is recycled for making paste again coal powder.
60% of
yield is obtained from this process.
7.
Explain the following (i) Compressed natural
gas (CNG) (ii) Liquid
petroleum gas. (LPG)
Compressed
Natural Gas: (CNG)
Natural
gas (CH4) compressed to a pressure of about 1000 atm is known as CNG. Its
calorific value is 12000-14000kcal/m3.
It is
fully of methane only & derived from natural gas. Its composition is as
follows.
CH4 = 70-90% C2 H2 = 5- 10 % H2 = 3%
Uses: CNG is
a cheapest, clearest & the least polluting fuel for automobiles instead of
petrol or diesel.
(ii) Liquid Petroleum Gas: (LPG)
It is
obtained as a by- product during the cracking of heavy oil. Its composition is
Butane =
27% Isobut ene = 25% Butyle nes = 43% Propan e = 5%
Its
calorific value is 27,800 kcal /m3.
LPG is
marketed under the trade names like Indane, HP, Bharat gas in steel cylinders
under high pressure.
A small
amount of Ethyl mercaptan is added during filling of cylinders to help in
detecting leakage of gas.
LPG
ensures complete combustion with no smoke & causes the least environmental
pollution.
Uses:
It is used as a domestic fuel.
It is used as a fuel in vehicles (i.e) motor fuel.
It is used in industries & laboratories
Explain
Water gas with reaction.
It is a
mixture of CO & H2 with small amount of N2.
Its
calorific value is about 2800kcal/m3.
Composition:
CO = 41%, H2 = 51%, N2 = 4% & CO2 = 4%.
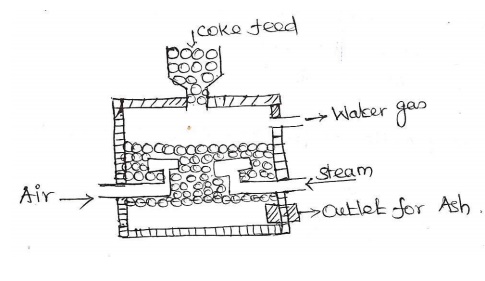
The water
gas producer consists of a tall steel vessel, lined with refractory bricks.
It is
provided with cup & cone feeder at the top & side opening for water gas
exists. At the bottom it is provided with 2 inlet pipes for passing air &
steam.
When
steam & little air is passed alternatively over a red hot coke maintained
at about 900-1000°C in a reactor, water gas is produced.
Two steps of reaction in production of water gas:
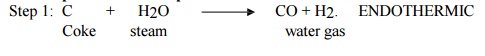
Here the
steam is passed through red hot coke, where CO & H2 gases are produced. The
reaction is endothermic.
Step 2: C + O2 --- > CO2. EXOTHERMIC.
Uses:
It is used for preparation of power alcohol.
For the production of H2 & in synthesis of NH3.
To manufacture synthesis petrol in Fischer – Tropsh
process.
Explain
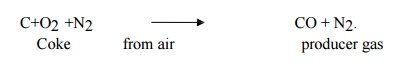
Producer gas with reaction.
It is a
mixture of CO & N2 with small amount of
H2. Its
calorific value is about 1300 kcal/m3.
Composition:
CO = 30%, H2 = 10-15%, N2 = 51-56% & others = rest.
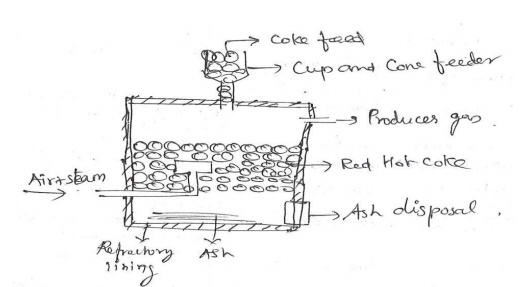
The
producer gas producer consists of a tall steel vessel, lined inside with
refractory bricks. It is provided with cup & cone feeder at the top &
side opening for producer gas exit.
At the
bottom it is provided with an one inlet for passing air & steam.
When a
mixture of air & steam is passed over a red hot coke at 1100°C in a
reactor, the producer gas is produced.
Uses:
It is a
used as a reducing agent in metallurgical operations.
Used for
heating muffle furnaces & open- hearth furnaces.
Describe
flue gas analysis by Orsat’s apparatus method.
Flue gas
analysis is carried out by Orsat apparatus method.
A mixture
of gases like CO2, CO, O2 & N2 etc coming out from the combustion chamber
is called flue gases.
If the
flue gas contains,
CO
-- It indicates incomplete combustion
&
O2 -- It
indicates excess supply of air used in combustion.
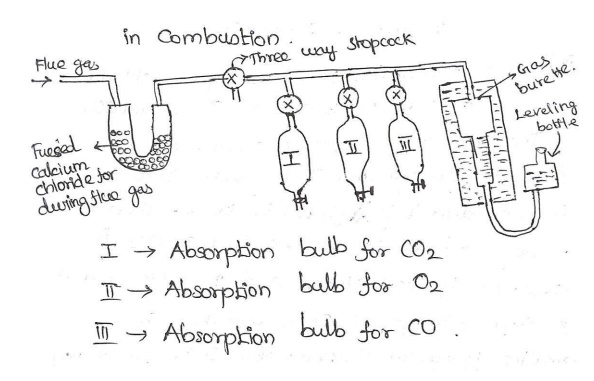
Apparatus: It consists a horizontal tube. At
one end this tube, U tube containing fused
CaCl2 is connected through 3 way stop cock. The other end is connected with
a graduated burette. The horizontal tube is also connected with 3 different
absorption bulbs I, II, III for absorbing CO2, CO and O2.
I bulb:
It contains KOH solutions & it absorbs CO2 only.
II bulb:
It contains Alkaline pyrogallol solution & it absorbs CO2, & O
III bulb:
It contains Ammoniacal cuprous chloride solution & it absorbs CO2,CO &
O
The 3 way
stop cock is connected with flue gas supply & it is sucked into the burette
& it is adjusted by 100cc. then the 3 way stop cock is closed. In bulb I,
co2 is absorbed by KOH solution & I is closed & II stopcock is opened,
O2 is absorbed by alkaline pyrogallol solution. Now II is closed & III is
opened. CO is absorbed by ammonical cuprous chloride. The decrease in volume of
the flue gas in the burette indicates the volume of I CO2, II O2,
III CO respectively.
Significance: It gives an clear idea about the
complete or incomplete process.
Related Topics