Chapter: Essential Clinical Immunology: Immunology of HIV Infections
Immunological and Biological Parameters of Disease
IMMUNOLOGICAL AND BIOLOGICAL PARAMETERS OF DISEASE
The pattern of disease progression
has now been well documented. Following infection with the virus, the virus
hones to and infects cells with CD4 receptors. During the early phase,
individuals may experience a flu-like illness with mild fever, cough, and
occasional chills. The symptoms subside, and the individual may be asymptomatic
for many years. In reality, the disease is progressing, and it is a long battle
between the immune response with production of new CD4+ cells and
the dying (apoptotic) HIV-infected CD4 cells. Eventually, the host immune
system deteriorates, and the individual succumbs to the complications secondary
to loss of the cellular immune system (see Figures 8.1 and 8.2). The pat-tern
and complications are quite similar to those seen in primary immunodeficiency
diseases. However, it has been a well-known observation that some individuals,
especially sex workers with repeated exposure to the virus, are rela-tively
resistant to HIV acquisition.
One of
the earliest markers in the progression of HIV-1 infection was the presence of
NEF in the viral strain. NEF is a 27–34 D myrisolated protein unique to primate
lente viruses. A functional NEF protein is important for the development of
high viremia and AIDS in simian immu-nodeficiency virus (SIV) in infected
rhesus
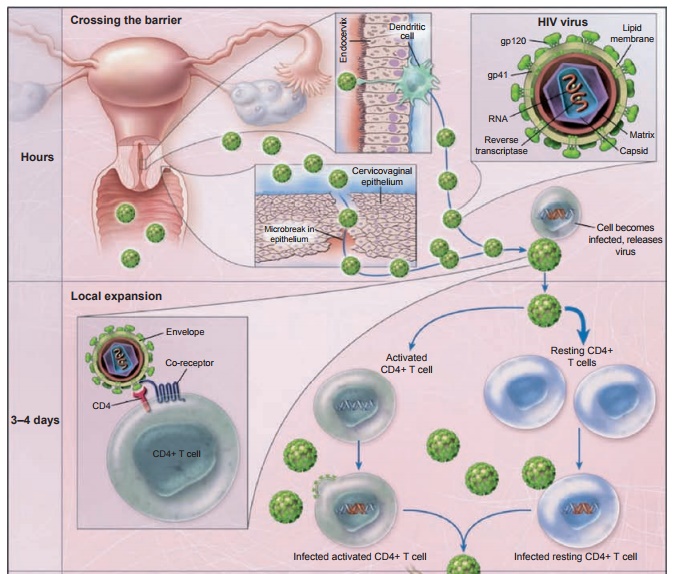
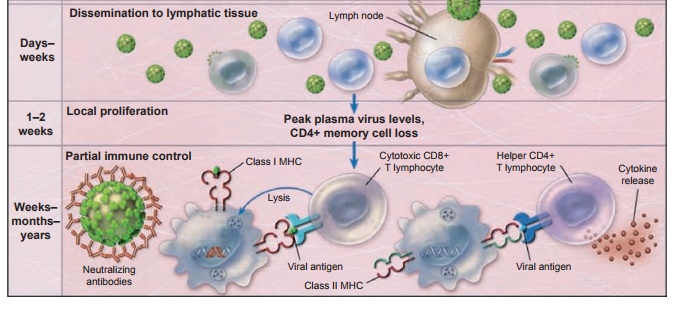
Figure 8.1 Early
events in the vaginal transmission of HIV, modeled after studies of simian immunodeficiency
virus infection in nonhuman primates. The binding of the HIV gp120 envelope
protein to CD4 T cells results in conformation change in the envelope,
interaction with the co-receptor, and fusion of the viral and cell membranes,
thus giving the HIV genome access to the interior of the cell. The infected
cells are carried first to draining lymph nodes and then spread systemically.
HIV-specific immune responses, including increases in CD8+ T
cells and eventually neutralizing antibodies, only partially control infection.
As a result, in the absence of effective vaccination or therapy, a slow and
continued depletion of CD4+ T cells ensues and there is a progression
to AIDS. Reprinted by permission from Macmillan Publishers: Haase AT. Perils at
mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792.
might decrease
the burst of viremia and dissemination that occurs in primary infection
(yellow), preserving gut-associated lymphoid tissue, diminishing the viral
reservoir, decreasing virus levels at the set point, and increasing the length
of time that viral levels are controlled (blue).
macaques. An important observation first noted in
the macaques and later in humans was that animals infected with NEF-deleted
virus were resistant to subsequent challenge with pathogenic wild-type viruses.
This was followed by the observation in humans that some individuals with
long-term non-progressive HIV-1 infection (persons who showed no clinical or
immunological signs of immunodeficiency despite being HIV seropositive for over
a decade) turned out to be infected with viruses carrying deletion in their NEF
gene.
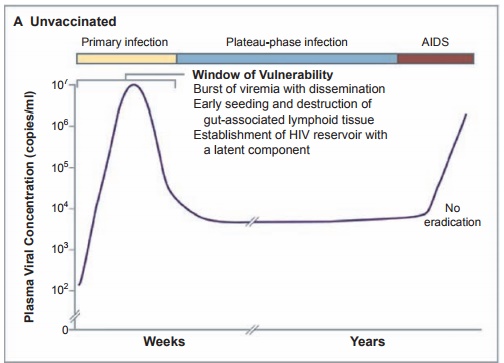
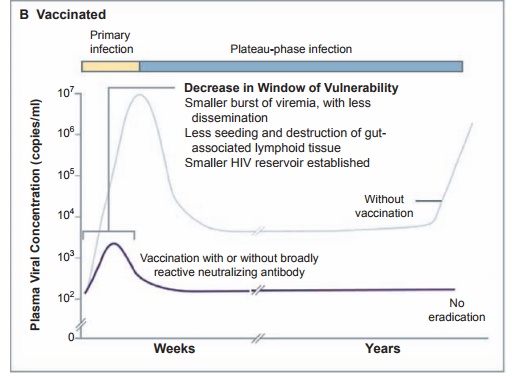
Figure 8.2 Panel A shows the course of infection in unvaccinated persons. The primary stage of HIV infection (yellow) starts with a burst of viremia, dissemination of the virus, early seeding and destruction of gut-associated lymphoid tissue, and establishment of a viral reservoir with a latent component. HIV levels in plasma then decline to a set point that lasts from months to years. Eventually, in the absence of effective therapy, the virus escapes immune control and AIDS results (red). Panel B shows the hypothetical course of infection in vaccinated persons. A T-cell vaccine
More recently, a large body of evidence has
appeared that shows that NEF has many important effects on many cells of the
immune system. Both exogenous and endogenous NEF down-regulates HLA-ABC
molecules critical for the initiation of cytotoxic T lymphocyte (CTL)
responses, thus impairing antigen presentation to HIV-specific CD8+
lymphocytes. NEF and gp120 also are able to down-regulate major
histocompatibility complex class I (MHC-I) in dendritic cells (DCs) and
introduction of exogenous NEF leads to up-regulation of MHC-II molecules,
thereby favoring CD4+ T-cell activation. This step increases the
“pool” of lymphocytes permissive to infection. However, endogenous NEF does not
modulate MHC-II surface expression; rather it induces a loss of co-stimulation.
These results underscore the pleiotrophic action of NEF. On one hand, exogenous
NEF triggers ABC-mediated bystander T-cell activation, ensuring viral spread,
while endogenous NEF induces a loss of co-stimulation, favoring immune evasion.
Another
area of interest has been the observation that NEF-pulsed DC produces a wide
variety of cytokines and chemokines typical of mature DC. This up-regulation of
cytokine production might promote bystander stimulation of T cells that cluster
around DC and could enhance HIV-1 replication in CD4+ T cells.
In
summary, the selective effects of the NEF protein may markedly enhance viral
pathogenesis and progression to disease. It does this by hijacking DC
functional activity and favoring HIV replication via bystander activation of
CD4+ cells and the escape of HIV-1 from immune surveil-lance by blocking CD8+
cells’ functional competence. Thus, efforts directed toward blocking NEF
influence in viral replication may have long-term beneficial effects on
therapeutic management of the disease.
Approximately
5–10 percent of sex workers in Kenya remained HIV unin-fected despite engaging
in unprotected sex with many male clients and the frequent acquisition of other
sexually transmitted infections. Because of the pioneering work of Plummer and
associates, the scientific reasons for this extraordinary resistance to the
virus in these women are beginning to unfold.
Turning
first to examination of CD4+ T-cell immune responses in seronegative
sex workers compared with HIV-positive women, CD4+ T cells that
produced inter-feron gamma (IFN-γ) in
response to HIV p24 were detected in exposed seronega-tive sex worker (ESN)
women, albeit at a much lower level than in HIV-positive women. However, ESN
women had a 4.5-fold stronger CD4+ proliferation response to the p24
peptide compared with the HIV-positive group. These data suggest that CD4+
T cells in ESN women recognize HIV and have an enhanced ability to proliferate
to the p24 protein.
Perhaps
more important has been the examination of the CD8+ cells in these
ESN individuals. Because IFN-γ
production cor-relates with cytotoxic functions, the CD8+ T
lymphocyte IFN-γ response to HIV p24 peptide in ESN women was
compared with HIV-positive individuals. Approximately 40 percent of ESN women
had a CD8+ IFN-γ-positive
response, and this was five times
lower in magnitude than that of HIV-posi-tive women. The breadth of the response
was very narrow and focused primarily on one peptide that is similar to KK10
protec-tive peptide when compared with the HIV-positive group. In HIV-positive
women, lower CD4+ counts influenced the num-ber of CD8+
cells producing IFN-γ, which
may undermine the ability to control HIV. These results indicate ESP women have
an HIV-1 p24 peptide-specific CD8+ IFN-γ response, providing evidence to
the speci-ficity needed for an effective HIV vaccine.
Further
studies of these women cen-tered around the known polymorphisms of the IL-4
gene and resistance to infection. Using microsatellite genotyping methods
coupled with the genomic sequencing, three polymorphisms in the interferon
reg-ulatory factor (IRF-1) located at
619a, 179, and 6516 of the gene, showed association with resistance to HIV
infection. Periph-eral blood mononuclear cells from these patients showed
significantly lower-based IRF-1 expression
and reduced respon-siveness to IFN-γ stimulation. This study added IRF-1, a transcriptional immunoreg-ulatory gene, to the list of
genetic correlates of altered susceptibility to HIV-1.
One of
the key cells in the immune sur-veillance system is the native DC, which is
well equipped for activation of both the innate and adaptive immune response.
It has been demonstrated that some DC-tropic viruses such as influenza virus
leave DC function intact while other viruses such as HIV and cytomegalovirus
have evolved strategies to impair DC functions, thereby enhancing the virus’s
ability to persist and escape immune surveillance.
DC
interaction with HIV is relevant to the pathogenesis of AIDS because they are
present in the mucosa and skin of humans and are believed to be the first HIV-1
tar-gets following sexual transmission of the virus. Both myeloid DC and
plasmacytoid DC possess the receptors for HIV entry; that is, CD4, CXR4, and
CCR5 can be infected but with a lower efficacy than CD4+ cells or
macrophages. The long-term result of this infection is that DC remains a
reser-voir for the production and persistence of the HIV-1 virus, and the virus
induces sev-eral functional impairments and variations in the DC population.
Thus, the number of both myeloid DC (MDC) and peripheral DC (PDC) are
significantly decreased in HIV-positive progressors, while remain-ing unaltered
in HIV-positive long-term nonprogression. The destruction of these cells may be
a consequence of direct lytic infection or as targets for specific CTL or
through a block in DC development from peripheral CD34+ stem cells.
More recent evidence supports the notion that both midi and pad show impaired
functional capacity in HIV-positive patients. Since both DC subsets participate
in the initiation of innate and adaptive immune responses, infection,
depletion, and dysfunction of DCs may contribute to the immunosup-pression seen
in HIV disease. Therefore, DCs play a dual role in HIV infection: they trigger
both innate and adaptic immune responses to control the infection, but they
also represent a viral reservoir for infection of permissive CD4+ T
cells.
Although the story of the African sex
workers who seem to be immune to HIV infection has captured the imagina-tion of
many researchers, there is another model of natural immunity that needs
examination in more detail. Most babies born to HIV-infected mothers also
escape infection even after potential intrauterine exposure and, more
important, exposure to virus-contaminating blood and secre-tions during labor
and delivery. Finally, breast-fed infants ingest hundreds of liters of
virus-infected breast milk. Many factors such as CD4 cell counts and viral load
in the secretions must be considered. One should add that, like the sex worker
model, it is a “real”-world situation of viral encounter opening a window to
the “in vivo” infection moment. Although interesting, there are, however,
several drawbacks to the model; notably, the newborn is immunologically
immature compared with later in life. However, their capacity to produce CC
cytokines is greater compared with their mothers whether she is HIV infected or
not (see Figure 8.3). This is consistent with the expected skewing toward a
stronger innate response rather than the adaptive response.
The
initially unexpected finding of HIV-specific CD4+ and CD8+
T-cell responses among uninfected sex workers paved the way to the fact that
natural protective immunity may exist. It has been difficult to repeat these
studies in infants because of
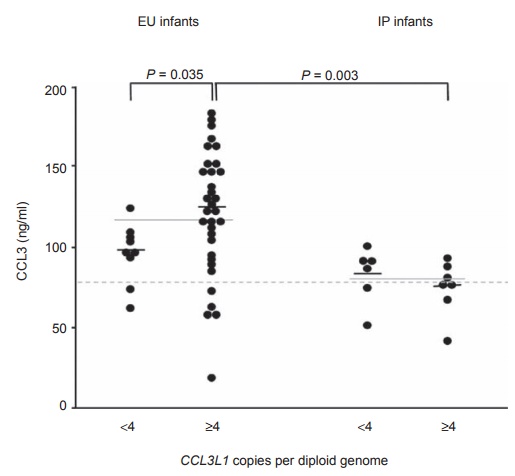
Figure 8.3 CCL3
production by cord blood cells. CCL3 concentrations in mitogen-induced
mononuclear cell cultures of umbilical cord blood from exposed and uninfected
(EU) and intrapartum-infected (IP) infants are stratified on the basis of low
numbers (less than 4) or high numbers (4 or more) of CCL3L1 copies per diploid
genome. Small, black horizontal lines, median values; solid gray lines across
low and high groups, median values for each total group; gray dashed line,
median value for uninfected controls. Reprinted by permission from Macmillan
Publishers: Tiemessen CT, Kuhn L. CC chemokines and protective immunity:
insights gained from mother-to-child transmission of HIV. Nat Immunol. 2007;8:219–222.
small blood volumes. Nevertheless, some studies,
using IL-2 production as a marker following stimulation with envelope peptides,
have shown that none of the children with apparent HIV-specific T-cell response
developed HIV infections despite exposure to HIV through breast-feeding.
However, this observation does not completely
explain why some babies are infected with HIV and others are not. In a landmark
study, Gonzalez and colleagues (2005) showed that segmental duplication
occurred in the gene CCL3L1-like chemo-kine. CCL3L1 as well as CCL3 are ligands
for CCR5 and are associated with lower susceptibility to HIV. Moreover, a study
of maternal-infant HIV transmission has shown that a phenotype of deficient
mito-gen-induced CCL3 production is associated with a greater risk of HIV
infection during labor and delivery. Thus the maternal-infant model has
demonstrated that the protein encoded for one or both of the functional genes (CCL3 or CCL3L1) is involved, sug-gesting that the abundance of this protein
may be important in HIV-protective immu-nity. Functional capacity, not just
gene cop-ies (four or more in some cases) of these pro-teins, is important.
This suggests that the “susceptibility phenotype” lives at the level of
induction of gene expression (see Figure 8.3) of CCL3 and CCL3L1,
resulting in quali-tative differences in protein production.
Turning to their use as vaccine candi-dates,
Tiemessen and colleagues (2007) suggest that, if CCL3 is a crucial molecule in
protection against HIV by virtue of its adjuvant ability, it may be useful as a
vac-cine adjuvant. In this manner, one does not have to rely on the host’s
ability to produce it (production may be deficient in certain populations; see
Figure 8.4).
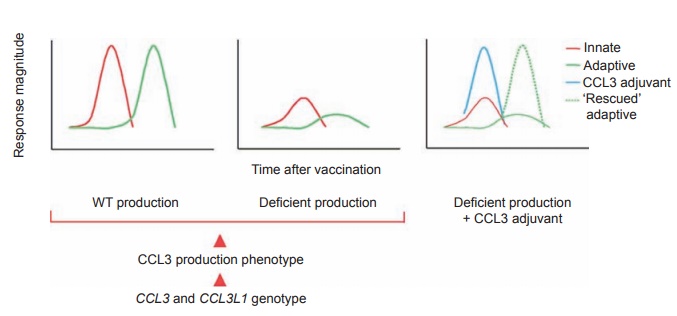
Figure 8.4 Possible
influence of CCL3 and CCL3L1 genotype on the
development of adaptive immunity to HIV
vaccines and restoration of loss of function in people with deficient
production through the provision of CCL3 as adjuvant. CCL3 and CCL3L1 genotypes
determine the production phenotype as wild type (WT) or deficient (red
arrowheads). Left, wild-type
production of CCL3 as the critical component of a rapid innate immune response
(red line) that “instructs” the effective development of subsequent adaptive
immunity (green line). Middle,
deficient production of CCL3 “translates” into an ineffective adaptive immune
response. Right, provision of CCL3 as
an adjuvant (blue line) compensates for deficient host production and restores
the development of adaptive immunity to wild-type capability (dashed green
line). Reprinted by permission from Macmillan Publishers: Tiemessen CT, Kuhn L.
CC chemokines and protective immunity: insights gained from mother-to-child
transmission of HIV. Nat Immunol.
2007; 8:219–222.
Innate immunity has been mostly neglected in favor of HIV-specific immunity in HIV vaccine studies. CCL3 production
should be considered a cor-relate of protective immunity. A desired response
would be sufficient production of CCL3 at the site of HIV and vaccine
encounter. It is imperative to delineate the precise functions of CCL3 in the
immune response to HIV, aside from its noncyto-lytic inhibitory effect on HIV,
as the abil-ity of CCL3 to drive the development of adaptive immunity might be
the crucial factor in overall protection. Many impor-tant questions arise,
given the findings outlined here. How should HIV vaccines be designed? How can
people who would be poor responders to vaccines (deficient CCL3 producers) be
identified? How can the genetically encoded loss of function be overcome, and
how can molecules be identified that can compensate for this? Innate immunity
must become a more integral component of studies of HIV vac-cines, as
understanding the interaction between innate and adaptive immunity may hold the
key to understanding what constitutes protective immunity to HIV. Studies of
uninfected infants born to HIV-infected mothers offer a unique human
experimental model for extending the understanding of such phenomena.
Related Topics