Chapter: social science geography student essay writing
If Emissions of Greenhouse Gases are Reduced, How Quickly do Their Concentrations in the Atmosphere Decrease?

If Emissions of
Greenhouse Gases are Reduced, How Quickly do Their Concentrations in the
Atmosphere Decrease?
The adjustment of greenhouse gas concentrations in the
atmosphere to reductions in emissions depends on the chemical and physical
processes that remove each gas from the atmosphere. Concentrations of some
greenhouse gases decrease almost immediately in response to emission reduction,
while others can actually continue to increase for centuries even with reduced
emissions.
The concentration of a greenhouse gas in the atmosphere
depends on the competition between the rates of emission of the gas into the
atmosphere and the rates of processes that remove it from the atmosphere. For
example, carbon dioxide (CO2) is exchanged between the atmosphere, the ocean
and the land through processes such as atmosphere-ocean gas transfer and
chemical (e.g., weathering) and biological (e.g., photosynthesis) processes.
While more than half of the CO2 emitted is currently removed from the
atmosphere within a century, some fraction (about 20%) of emitted CO2 remains
in the atmosphere for many millennia. Because of slow removal processes,
atmospheric CO2 will continue to increase in the long term even if its emission
is substantially reduced from present levels. Methane (CH4) is removed by
chemical processes in the atmosphere, while nitrous oxide (N2O) and some
halocarbons are destroyed in the upper atmosphere by solar radiation. These
processes each operate at different time scales ranging from years to
millennia. A measure for this is the lifetime of a gas in the atmosphere,
defined as the time it takes for a perturbation to be reduced to 37% of its initial
amount. While for CH4, N2O, and other trace gases such as
hydrochlorofluorocarbon-22 (HCFC-22), a refrigerant fluid, such lifetimes can
be reasonably determined (for CH4 it is about 12 yr, for N2O about 110 yr and
for HCFC-22 about 12 yr), a lifetime for CO2 cannot be defined.
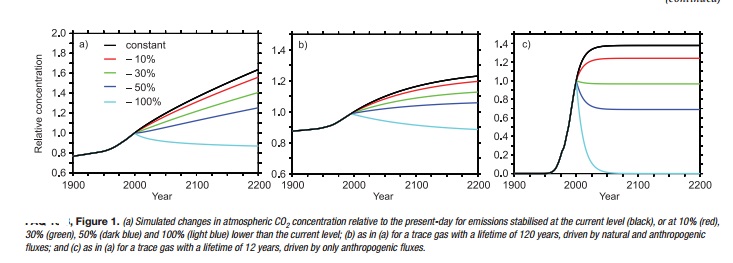
The change in concentration of any trace gas depends in part
on how its emissions evolve over time. If emissions increase with time, the
atmospheric concentration will also increase with time, regardless of the
atmospheric lifetime of the gas. However, if actions are taken to reduce the
emissions, the fate of the trace gas concentration will depend on the relative
changes not only of emissions but also of its removal processes. Here we show
how the lifetimes and removal processes of different gases dictate the
evolution of concentrations when emissions are reduced.
As examples, FAQ 10.3, Figure 1 shows test cases illustrating
how the future concentration of three trace gases would respond to illustrative
changes in emissions (represented here as a response to an imposed pulse change
in emission). We consider CO2, which has no specific lifetime, as well as a
trace gas with a well-defined long lifetime on the order of a century (e.g.,
N2O), and a trace gas with a well-defined short lifetime on the order of decade
(such as CH4, HCFC-22 or other halocarbons). For each gas, five illustrative
cases of future emissions are presented: stabilisation of emissions at
present-day levels, and immediate emission reduction by 10%, 30%, 50% and 100%.
The behaviour of CO2 (Figure 1a) is completely different from
the trace gases with well-defined lifetimes. Stabilisation of CO2 emissions at
current levels would result in a continuous increase of atmospheric CO2 over
the 21st century and beyond, whereas for a gas with a lifetime on the order of
a century (Figure 1b) or a decade (Figure 1c), stabilisation of emissions at
current levels would lead to a stabilisation of its concentration at a level
higher than today within a couple of centuries, or decades, respectively. In
fact, only in the case of essentially complete elimination of emissions can the
atmospheric concentration of CO2 ultimately be stabilised at a constant level.
All other cases of moderate CO2 emission reductions show increasing concentrations
because of the characteristic exchange processes associated with the cycling of
carbon in the climate system.
More specifically, the rate of emission of CO2 currently
greatly exceeds its rate of removal, and the slow and incomplete removal implies
that small to moderate reductions in its emissions would not result in
stabilisation of CO2 concentrations, but rather would only reduce the rate of
its growth in coming decades. A 10% reduction in CO2 emissions would be
expected to reduce the growth rate by 10%, while a 30% reduction in emissions
would similarly reduce the growth rate of atmospheric CO2 concentrations by
30%. A 50% reduction would stabilise atmospheric CO2, but only for less than a
decade. After that, atmospheric CO2 would be expected to rise again as the land
and ocean sinks decline owing to well-known chemical and biological
adjustments. Complete elimination of CO2 emissions is estimated to lead to a
slow decrease in atmospheric CO2 of about 40 ppm over the 21st century
The situation is completely different for the trace gases
with a well-defined lifetime. For the illustrative trace gas with a lifetime of
the order of a century (e.g., N2O), emission reduction of more than 50% is
required to stabilise the concentrations close to present-day values (Figure
1b). Constant emission leads to a stabilisation of the concentration within a
few centuries.
In the case of the illustrative gas with the short lifetime,
the present-day loss is around 70% of the emissions. A reduction in emissions
of less than 30% would still produce a short-term increase in concentration in
this case, but, in contrast to CO2, would lead to stabilisation of its
concentration within a couple of decades (Figure 1c). The decrease in the level
at which the concentration of such a gas would stabilise is directly
proportional to the emission reduction. Thus, in this illustrative example, a
reduction in emissions of this trace gas larger than 30% would be required to
stabilise concentrations at levels significantly below those at present. A
complete cut-off of the emissions would lead to a return to pre-industrial
concentrations within less than a century for a trace gas with a lifetime of
the order of a decade.
Related Topics