Chapter: Essentials of Psychiatry: Pharmacotherapies for Substance Abuse
Drug Treatment of Withdrawal Syndromes
Drug
Treatment of Withdrawal Syndromes
Some
general principles apply to drug treatments for specific withdrawal syndromes:
When
monitoring treatment:
·
Set clear targets.
·
Make serial assessments and modify based on these
assessments.
Psychosocial
factors:
·
Prepare the patient.
·
Emphasis on detoxification as a beginning to
treatment.
From a
pharmacologic standpoint, an ideal agent for the treat-ment of withdrawal
should have the following characteristics:
·
Efficacy in relieving the complete range of
abstinence signs and symptoms for a given type of withdrawal.
·
A relatively long duration of action and gradual
offset of effects.
·
A high degree of safety in the dosage needed to
suppress with-drawal (i.e., high therapeutic index).
·
It should be available by a variety of routes of
administration and have little abuse potential in itself.
Other
general but important aspects of detoxification are also fre-quently
overlooked:
·
Clear treatment targets should be kept in mind.
·
Structured rating scales should be used to measure
symptom severity: serial assessment of the clinical response is the best
strategy for guiding treatment and detoxification should not be conducted “on
autopilot”.
·
While protocols may offer useful guidance, orders should
be rewritten with thought and often daily.
Patients
must understand that detoxification is not a treatment for addiction but the
beginning of treatment for the chronic problems associated with substance
dependence. They should know what they will experience and they should if at
all possible be engaged in the effort to relieve their symptoms as safely as
possible and with greatest effectiveness. They need to understand, however,
that they are likely to experience some distress. Detoxification can be carried
out on an outpatient basis for patients who are in relatively good health and
have sufficiently stable social support.
Alcohol Withdrawal
Alcohol
withdrawal is a medical emergency and can be life-threatening without
appropriate supportive medical treatment.
The risk
of progressing from uncomplicated withdrawal to seizures or delirium should be
greatly reduced by proper man-agement. Patients should be assessed
systematically when enter-ing the detoxification protocol and during the
process. A widely used but simple tool is the Clinical Institute Withdrawal
Assess-ment – Alcohol Scale (Table 84.1). Starting 6–24 hours after the last
drink, this scale should be used to assess the patient initially every 1–2
hours and at regular intervals until two consecutive scores below 8–10 are
achieved. It is then safe to end structured assessment. Scores greater than 15
are an indication for monitor-ing the patient even more closely. Scores in
between these limits should be interpreted according to clinical judgment
according to the degree of discomfort reported by the patient and their history
of withdrawal syndromes.


Undertreatment
with doses that are too low or dose inter-vals that are too long is the
commonest error in the drug treat-ment of withdrawal syndromes. However,
striking the balance between withdrawal and intoxication is not complicated
provided serial assessments are made.
Benzodiazepines
are the drugs of choice for treating alco-hol withdrawal because:
·
There is cross-tolerance with alcohol (therefore
higher than normal doses may be needed).
·
They are relatively safe compared with other
sedative-hypnotics.
·
They have been shown to reduce the frequency of
seizures and delirium.
The
benzodiazepines can be divided into two major classes according their duration
of action. Longer-acting agents, includ-ing chlordiazepoxide and diazepam,
undergo metabolic oxidation and glucuronidation. Their advantage is that blood
levels decline more slowly during the tapering process, increasing patient
com-fort and reducing the risk of seizures. The disadvantage is that, in older
patients and patients with impaired hepatic or pulmonary function, decreased
elimination may result in accumulation and toxicity. Chlordiazepoxide may be
preferred to diazepam because it may have a lower abuse potential.
Shorter-acting
agents, such as oxazepam and lorazepam, are metabolized only by
glucuronidation. Both drugs are available in oral and intravenous forms though
only lorazepam may also be administered by intramuscular injection. These
agents are more readily metabolized and eliminated by older patients and they
are less likely to accumulate and cause toxicity in patients with liver
disease. However, blood levels decline more rapidly and this may cause breakthrough
symptoms and seizures between doses.
The
treatment response should be assessed serially. Medi-cation should adjusted
according to clinical need: if signs of
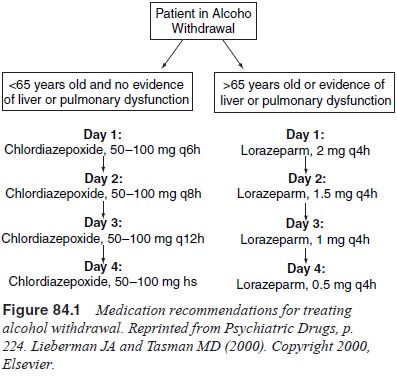
withdrawal
are apparent, the dose should be increased or the dose interval reduced. Some
degree of sedation is desirable but medi-cation should be withheld if the
patient becomes over-sedated and recommenced when clinically indicated.
Recommendations for medication are summarized in Figure 84.1.
Hallucinations
that develop as a feature of delirium are relatively refractory to treatment
with a benzodiazepine alone. Patients who develop hallucinations despite
sedative-hypnotic substitution may be treated with adjunctive haloperidol, 2 to
5 mg.
In the
event of persistent tachycardia or hypertension, the possibility of
inadequately treated withdrawal should first be ex-cluded. This problem has
been managed successfully with beta-blockers and clonidine but while these
agents may decrease vital signs and tremor they do not prevent seizures.
An
alternative strategy is to “frontload” the patient, achieving sedation by
administering diazepam or chlordiazepox-ide every 1 to 2 hours. Because these
agents have a long elimina-tion half-life, their effects diminish slowly and
smoothly over the withdrawal period. However, careful serial assessment remains
essential while the patient is vulnerable to the complications of withdrawal.
Thiamine
is indicated for every patient with alcoholism to prevent Wernicke’s
encephalopathy and Korsakoff’s amnestic syndrome. It should be administered
immediately (typical dose 100 mg daily), before intravenous glucose. Less
urgently, nutri-tional support should include supplementation with folate 1 mg
and multivitamins. It has been suggested that magnesium sup-plementation may
prevent withdrawal seizures but there is no consensus on its use in the absence
of documented magnesium deficiency.
There are
promising reports on the use of carbamazepine and valproate in alcohol
withdrawal and they may be helpful in special circumstances. However, the
safety and efficacy of ben-zodiazepines are currently unsurpassed.
Sedative-hypnotic Withdrawal
Similar
to the treatment for alcohol withdrawal, benzodiazepine taper (as described
above) is a good choice, particularly if the patients is dependent on
benzodiazepines. Another excellent choice for substitution therapy is
phenobarbital: it has low po-tential for abuse, there is a wide margin between
therapeutic and lethal blood levels, and it has a long duration of action with
rela-tively little variation in between-dose blood levels. The symp-toms of
intoxication with phenobarbital (ataxia, slurred speech, nystagmus) are readily
observed easy and managed within a de-toxication protocol.
For
patients dependent on sedative-hypnotics, the first step in management if to
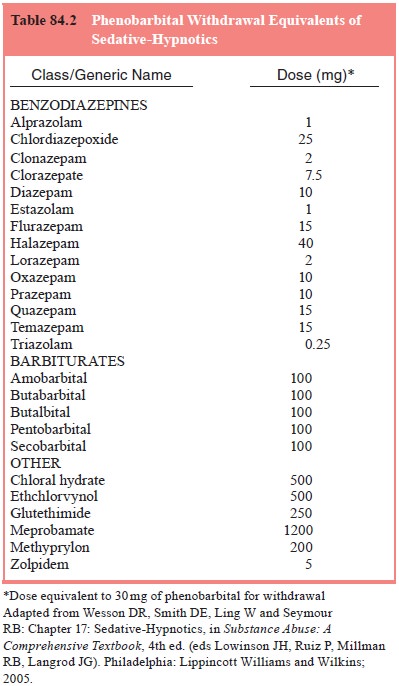
take a history. Information about the level of drug consumption can be
converted to “phenobarbital equiva-lents” to provide a guide to the required
dose of phenobarbital, as specified in Table 84.2.
The
number of phenobarbital equivalents is summed and administered as a
thrice-daily dose regimen. The total daily requirement of phenobarbital rarely
exceeds 500 mg even in patients with extreme dependency. The first dose of
phenobarbital may be administered as an intramuscular injec-tion if signs of
withdrawal occur before substitution therapy begins. Symptoms of withdrawal and
intoxication should be reassessed after 1 to 2 hours to determine the next dose
of phenobarbital.
The
degree of dependence can also be assessed by serial challenge with
pentobarbital at doses of 200 mg but it is not clear that this method offers
any advantage over direct substitution with phenobarbital, which is safe, rapid
and simple.
There
should be no signs of sedative-hypnotic withdrawal or phenobarbital toxicity
after 24 to 48 hours; dose reduction of phenobarbital can then be started.
Maintaining the thrice-daily dose regimen, the dose of phenobarbital should be
reduced in increments of 30 mg/day. If there is evidence of phenobarbital
toxicity (slurred speech, nystagmus, ataxia), the next dose should be withheld
and the total daily dose reduced. Conversely, if there are objective signs of
withdrawal, the total daily dose of pheno-barbital should be increased and dose
reduction delayed until the patient is once again stable.
Opiate Withdrawal
Withdrawal
from opiates may be intensely uncomfortable but, by contrast with
sedative-hypnotic withdrawal, it is not usually life-threatening for adults;
important exceptions include adults with little reserve – for example, due to
to advanced AIDS – and newborn infants. Nevertheless, reducing withdrawal
symptoms may help to engage an addict in a treatment program or facilitate the
management of another medical condition.
Methadone
is approved by the FDA for treating opiate withdrawal but states may have
different regulations governing its use and clinicians need to be aware of
local requirements. Methadone use is typically permitted for inpatient
detoxification or maintenance treatment but it cannot be prescribed to treat
opi-ate withdrawal in outpatients except as part of a licensed metha-done
maintenance treatment program.
Methadone
is administered orally and has a long duration of action. An initial dose of 15
to 20 mg may be given when signs of opiate withdrawal are seen (not merely when
craving is re-ported). An additional 5 to 10 mg may be given in 1 to 2 hours if
symptoms persist or worsen. A dose of 40 mg/day usually con-trols signs of
withdrawal well (note that this is often insufficient for the different
indication of long-term maintenance). If oral ad-ministration is impossible due
to withdrawal symptoms, doses of 5 mg may be administered by intramuscular
injection. Having reached a dose at which withdrawal symptoms are alleviated,
the dose can be tapered by 5 to 10% per day until full detoxification is
achieved.
A newly
available option for treatment of opioid with-drawal is the Schedule III opioid
partial agonist buprenorphine, which is now available for use in the office
based practice by any physician who has taken a brief training and
certification. While the use of Schedule II drugs such as methadone for the
treat-ment for opiate dependence is restricted to hospitals or specially
licensed clinics (leading to a shortage of facilities where opiate-dependent
individuals could receive appropriate treatment), the Drug Addiction Treatment
Act of 2000 introduced less stringent regulations, allowing the use of narcotic
drugs for the treatment of addiction in the office or any other health care
setting by any licensed physician who has taken a brief training course and
obtained registration, thereby increasing access to treatment. Two new
formulations of the buprenorphine (Subutex and Sub-oxone sublingual tablets)
were the first products to be approved by the FDA under this Act for the
treatment of opioid depend-ence. Subutex contains only buprenorphine in doses
of 2 or 8 mg; Suboxone also contains the opioid antagonist naloxone (0.5 and 2
mg respectively); the purpose of the naloxone is to discourage diversion of the
medication for abuse intravenously (crushing the pills and injecting them),
since naloxone is poorly absorbed after oral or sublingual administration, but
if injected would produce precipitated withdrawal.
Buprenorphine
is an excellent detoxification agent because of its long duration of action,
due to very high affinity for and very slow dissociation from opioid receptors.
Starting buprenorphine must be done carefully, because of the partial agonism.
If admin-istered too close in time to the last dose of a full agonist such as
heroin, buprenorphine will precipitate withdrawal, and the with-drawal produced
can be atypical and in rare cases has been ob-served to include delirium.
Precipitated withdrawal is more likely among patients dependent on long-acting
agonists (e.g. metha-done), or high daily doses of a shorter-acting agonist
such as heroin. Thus, when starting buprenorphine, the clinician should wait
for symptoms of opioid withdrawal to begin to appear before giving the first
dose of buprenorphine, which should be a test dose of 2 mg (Subutex 2 mg, or
Suboxone (2 mg buprenorphine/0.5 mg naloxone). If this dose is well tolerated,
administer another 2 mg one hour later, and up to 8 mg total on the first day, and
up to 16 mg on the second day. After this buprenorphine can be tapered slowly
to zero over 10 days to 2 weeks. Considerable flexibility in the taper schedule
is possible, including a much faster taper, since the slow dissociation from
receptors in itself effectively produces a taper. However, clinicians should
also be alert for the emergence of low-grade withdrawal symptoms (fatigue,
anxiety, mild flu-like physical symptoms) in the weeks after discontinuing
buprenor-phine. This subacute or protracted withdrawal can be observed after
withdrawal from any opioid drug, but can seem surprisingly with buprenorphine
because the taper phase of a buprenorphine detoxification is usually
comfortable and uneventful.
If one of
the first few doses of buprenorphine administered is followed by a rapid
worsening of withdrawal symptoms, this is precipitated withdrawal, and no
further buprenorphine should be given; at this point it is probably best to
treat with a full agonist (methadone), although one could also wait for precipitated
with-drawal to clear and full-blown opiate withdrawal (from whatever the
patient was addicted to) to emerge, after which one can try again beginning
with a test dose of 2 mg buprenorhine.
A number
of nonnarcotic medications are useful in treat-ing the symptoms of opiate
withdrawal. These include the α-adrenergic
receptor agonist and antihypertensive clonidine, which is
particularly helpful with the autonomic symptoms of withdrawal as well as the
anxiety, benzodiazepines (clonazepam is typically used), which are particularly
helpful for the anxi-ety and insomnia, antiemetics and NSAIDs for muscle aches
(oral agents such as ibuprofen, or Toradol which can be given parenterally).
Autonomic symptoms may be well controlled with clonidine alone but patients
frequently report greater subjective distress than with methadone or
buprenorphine. The role of cloni-dine is for detoxification from illegal
opiates, for example in set-tings where methadone is not allowed, or to
alleviate abstinence symptoms when a patient comes to the end of a methadone
main-tenance program. Its major side effects are hypotension (which may require
dose reduction or discontinuation) and sedation. Hypotension may be worsened by
diarrhea, or vomiting, which are common in opiate withdrawal. Patients should
be encouraged to take plenty of fluids, and sports drinks such as gatorade are
particularly helpful because they also supply electrolytes. Clo-nidine does not
address all aspects of withdrawal and it should therefore be used in
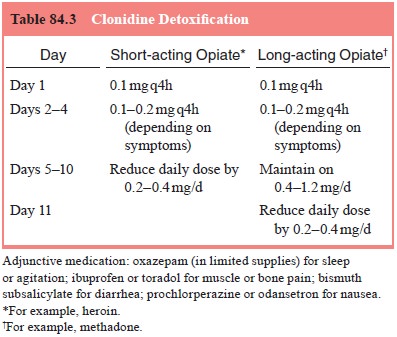
combination with the alternatives detailed above. A protocol for
clonidine-assisted detoxication is given in Table 84.3.
A
combination of clonidine with naltrexone has been used to achieve a more rapid
withdrawal, followed by maintenance treatment with naltrexone. However,
clinical experience and close monitoring of the patient is necessary to titrate
the dose of clonidine against withdrawal symptoms induced by naltrexone. The
acceptability of maintenance treatment with naltrexone to opiate addicts is
disappointing.
With
opiate detoxification, it is particularly important to consider the indications
for it, and to establish an adequate treatment plan after detoxification is
completed. Chronic opioid use induces tolerance, and detoxification reduces or
eliminates tolerance. Because of the loss of tolerance, detoxified opiate
ad-dicts are at increased risk for death from opiate overdose; doses that they
routine self-administered previously when tolerant, could now be lethal, a
problem exacerbated by the variable and sometimes high potency of illicit
heroin. In general, the risk of re-lapse following opioid detoxification is
high. Therefore, patients should be assessed for overdose risk, and those with
a history of past overdoses, or with multiple past relapses, should be
encour-aged to take agonist maintenance treatment, with methadone or
buprenorphine, rather than undergoing detoxification. For those not entering
agonist maintenance, a strong plan for psychosocial treatment is important, for
example long-term residential treat-ment or Therapeutic Community, a good
outpatient treatment program, supplemented by self-help group (Alcoholics
Anony-mous, or Narcotics Anonymous).
Related Topics