Measurement | Term 1 Unit 1 | 7th Science - Density | 7th Science : Term 1 Unit 1 : Measurement
Chapter: 7th Science : Term 1 Unit 1 : Measurement
Density
Density
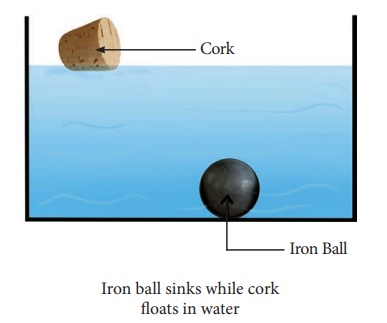
Take water in a beaker and drop an
iron ball and a cork bowl into the water. What do you observe? The iron ball
sinks and the cork floats as shown in figure. Can you explain why? If your
answer is “heavy objects sink in water and lighter objects
float is water”,
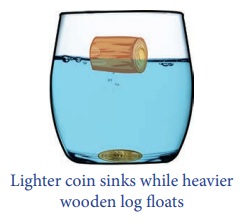
then, why does a metal coin sink in water whereas a much heavier wooden log
floats? These questions can be answered when we understand the concept of
density.
ACTIVITY 3
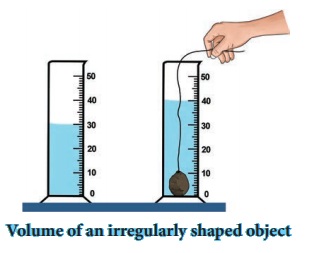
Take a measuring cylinder and pour some water into it (Do
not fill the cylinder completely). Note down the volume of water from the
readings of the measuring cylinder. Take it as V1. Now take a small
stone and tie it with a thread. Immerse the stone inside the water by holding
the thread. This has to be done such that the stone does not touch the walls of
the measuring cylinder (Figure). Now, the level of water has raised. Note down
the volume of water and take it to be V2. The volume of the stone is
equal to the raise in the volume of water.
V1 = 30CC ; V2 = 40 CC;
Volume of stone = V2 – V1
= 40 – 30 = 10 CC.
From the activity 4, we observe that
wooden block occupies more volume than the iron ball of same mass. Also, we
observe that wooden block is lighter than the iron block of same size.
The lightness or heaviness of a body
is due to density. If more mass is packed into the same volume, it has greater
density. so , the iron block will have more mass than the wooden block of the
same size. Therefore iron has more density.
Definition of density:
Density
of a substance is defined as the mass of the substance contained in unit volume
(1 m3).
If
the mass of a substance is “M” whose volume is “V”, then, the equation for
density is given as
Density (D) = mass(M) / Volume(V )
D = M / V
ACTIVITY 4
a. Take an iron block
and a wooden block of same mass (say 1kg each). Measure their volume. Which one
of them has more volume and occupies more volume?
Answer: Wooden block
b. Take an iron block
and a wooden block of same size. Weigh them and measure their mass. Which one
of them has more mass?
Answer: iron block
Unit of density
SI unit of density is kg/m3.
The CGS unit of density is g/cm3.
Density of different materials
Different materials have different
densities. The materials with higher density are called “denser” and the materials
with lower density are called “rarer”.
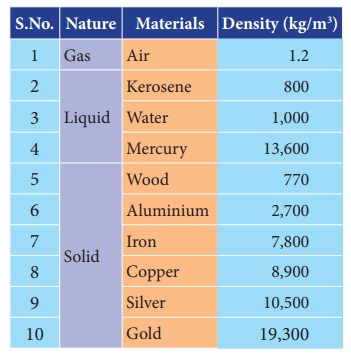
The density of some widely used materials are listed in the following table 1.4.
Table 1.4 Density of some common substances, at room temperature
Suppose you have one Kg of iron and
gold, which of them would have more volume than the other? Give your reason.
Problem 1.4
A solid cylinder of mass 280 kg has
a volume of 4 m3. Find the density of cylinder.
Solution:
Density
of cylinder = mass of
cylinder / volume of cylinder
=280/4 = 70 kg/m3
Problem 1.5
A box is made up of iron and it has
a volume of 125 cm3. Find its mass. (Density of iron is 7.8 g / cm3).
Solultion:
Density =Mass / Volume
Hence, Mass =Volume × Density
=125 × 7.8 = 975 g.
Problem 1.6
A sphere is made from copper whose
mass is 3000 kg. If the density of copper is 8900 kg/m3, find the
volume of the sphere.
Solution:
Density = Mass / Volume
Hence, Volume = Mass / Density
= 3000 / 8900 = 30 / 89
=0.34 m3
The relationship between Mass,
density and volume are represented in the following density triangle:
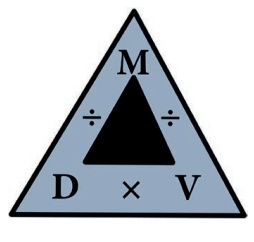
*
Density= Mass/ Volume
*
Mass= Density × Volume
*
Volume= Mass / Density
Relationship between density, mass
and volume
Related Topics