Chapter: Obstetrics and Gynecology: Cell Biology and Principles of Cancer Therapy
Chemotherapy
CHEMOTHERAPY
Chemotherapeutic
agents can be (1)
cell cycle (phase)-nonspecific, which means that they can kill in all
phases ofthe cell cycle and are useful in tumors with a low growth index, or
(2) cell cycle (phase)-specific,
which means that they kill in a specific phase of the cell cycle and are most
use-ful in tumors that have a large proportion of actively divid-ing cells.
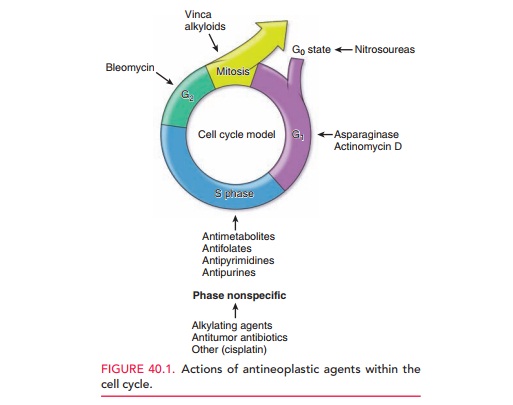
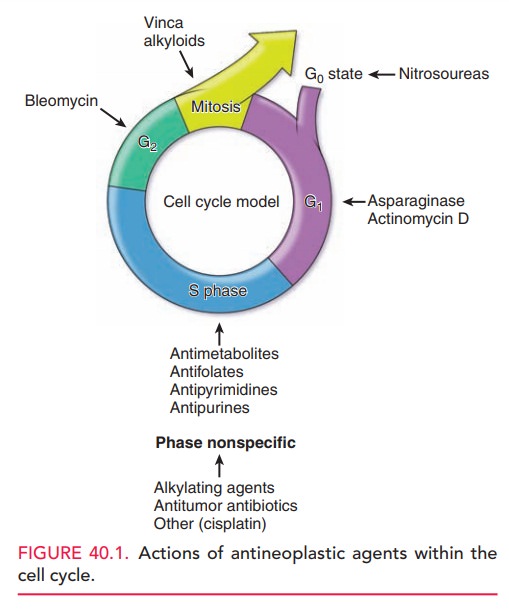
Figure 40.1 illustrates common drugs and their sites of action within the cell
cycle.
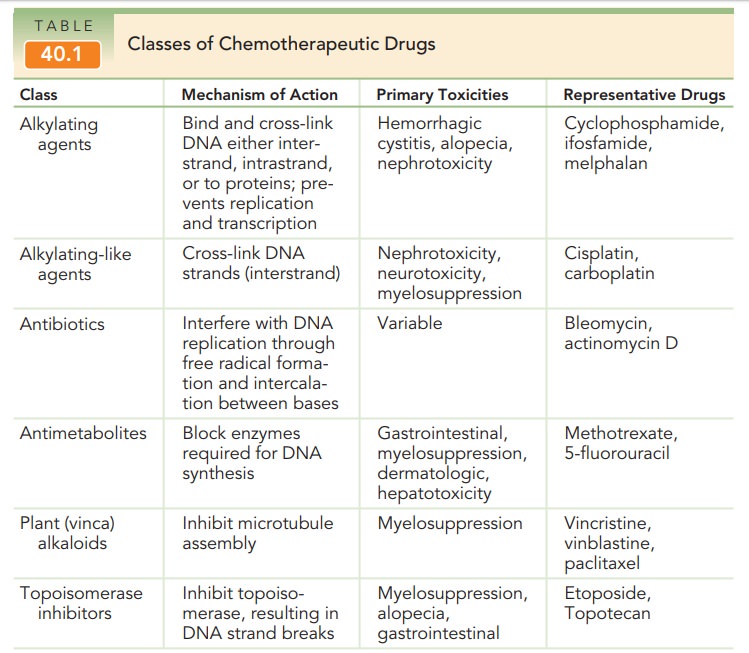
Several classes of antineoplastic drugs are available (Table 40.1). Alkylating agents and alkylating-like agents bind and cross-link DNA, interfering with DNA replica-tion and, ultimately, with RNA transcription. Dividing cells, especially those in the late G1 and S phases, are most sensitive to the effects of these drugs; however, these drugs are considered phase-nonspecific (i.e., they are effective in all phases of the cell cycle). The major side effect of the alkylating agents is myelosuppression. The alkylating-like agents behave similarly, and include the platinum-based agents cisplatin and carboplatin.
Antitumor
antibiotics inhibit DNA-directed RNAsynthesis and also are
involved in the formation of free rad-icals, causing strand breakage. They are
phase-nonspecific. Their general side effects are similar to those of the
alky-lating agents; however, each individual drug has its own individual
toxicity.
Antimetabolites
are structural analogs of normalmolecules necessary
for cell function. They competi-tively interfere with the enzymes involved with
normal synthesis of nucleic acids and, therefore, are most active during the S
phase of cell division. They may cause bone marrow suppression or
gastrointestinal mucositis when given in a bolus.
Plant
(vinca) alkaloids interfere with the M phaseof cell
division by preventing the assembly of micro-tubules. They may cause bone
marrow suppression or an anaphylactoid reaction.
Topoisomerase
inhibitors result in cell death byinhibiting topoisomerase I
(TOPO-I), an enzyme required for DNA replication. In a normally replicating
cell, TOPO-I induces reversible single-strand breaks in the DNA. TOPO-I
inhibitors complex with the DNA and TOPO-I and prevent repair of the breaks in the
single strand of DNA, thus resulting in cellular death.
Endocrine therapy with selective estrogen receptormodulators (SERMs)
acts in estrogen-sensitive breasttumors to block the interaction of estrogen
with estrogen receptors (ERs). The therapeutic importance of cellular ERs has
been well established in breast cancers. ER-positive tumors are responsive to
endocrine therapy. Normally, estrogen enters cells and binds to ERs in the
cytoplasm. The complex is translocated to the nucleus, where it binds to acceptor
sites on chromosomes, resulting in activation of RNA and protein synthesis.
SERMs act as competitive inhibitors of estrogen binding; the SERM–ER complex
binds to chromosomes, but does not activate cell metab-olism. The subsequent
decrease in cellular activity and cell division results in reduced tumor
growth. The two SERMs most frequently prescribed in the United States are
tamoxifen and raloxifene. Although relatively nontoxic, this class of drugs is
related to an increased risk of endometrial cancer and uterine sarcomas and an
increase in benign endometrial pathology.
Aromatase
inhibitors (AIs), which suppress intra-tumor and plasma
estrogen levels, are being used in post-menopausal patients for treatment of
advanced breast cancer that has progressed beyond tamoxifen therapy.
Progestational
agents have been found to be use-ful in the treatment of
early-stage endometrial cancer when surgery is either not feasible, unsafe, or
not desired. Progestational therapy is also useful for some patients with recurrent
disease. The most common progestational agents used are medroxyprogesterone and
megestrol. Other hormonal agents that have demonstrated efficacy in cases of
recurrent disease include tamoxifen (SERM), gosere-lin (synthetic hormone),
anastrozole (AI), and arzoxifene (SERM).
Antineoplastic
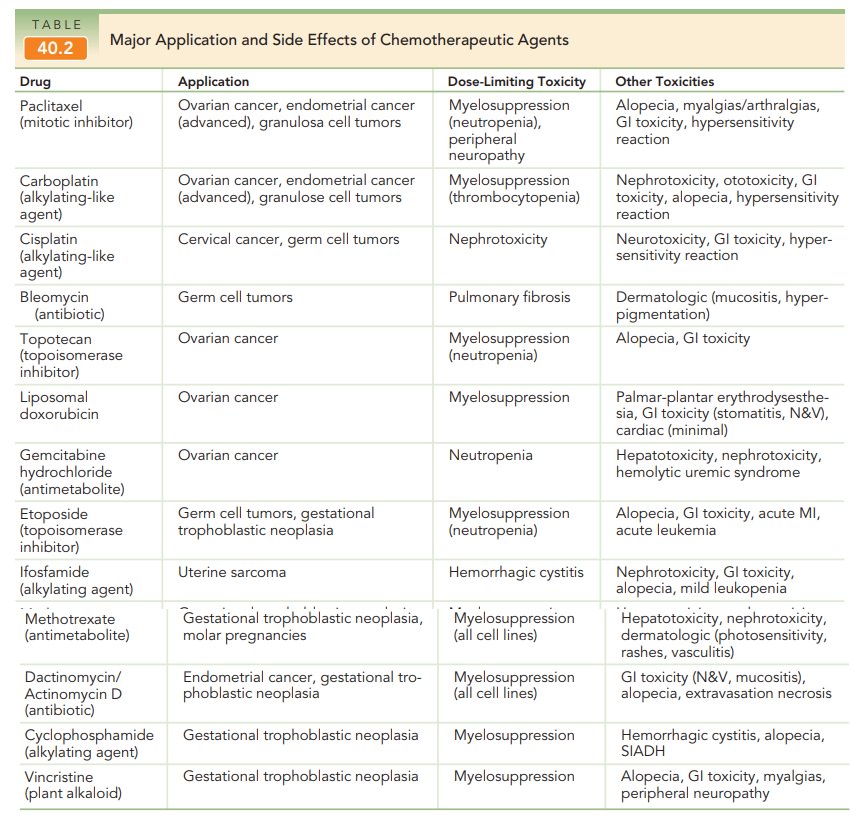
drugs are toxic because they act on normal as well as cancer cells. Table
40.2 describes the major applica-tions and side effects of antineoplastic
agents. Rapidly dividing cell types of the erythroid, myeloid, and
megakary-ocytic lineages are most sensitive to damage by common neoplastic
drugs. Anemia, granulocytopenia (neutropenia), and thrombocytopenia are
predictable side effects. Patients with anemia will often experience
incapacitating lethargy. Patients with neutropenia are at high risk for fatal
sepsis, and those with sustained thrombocytopenia are at risk for spontaneous
gastrointestinal or acute intracranial hemor-rhage. Prophylactic antibiotics
are administered to patients with febrile neutropenia or in neutropenic
patients to pre-vent serious infection. Platelet transfusions can be used to
decrease the risk of hemorrhage.
The use of single agents is
limited by development of drug resistance and toxicity. Combination chemotherapy is used to counteract these limitations.
Several strategies can be used to select drugs for combination chemotherapy. In
sequential blockade, the drugs block
sequential enzymesin a single biochemical pathway. In concurrent blockade, the drugs attack parallel biochemical pathways
leading to the same end product. Complementary inhibition inter-feres with
different steps in the synthesis of DNA, RNA, or protein.
The interactions between drugs
used in combination are defined as synergistic
(result in improved antitumor activity or decreased toxicity, compared with
when each agent is used alone), additive
(result in enhanced antitu-mor activity equal to the sum of the antitumor
activities resulting from using the individual agents separately), or antagonistic (result in less antitumor
activity than if eachindividual agent is used alone). Drugs used in
combinations should (1) be effective when used singly, (2) have different
mechanisms of action, and (3) be additive or, preferably, synergistic in
action.
Chemotherapy
is administered in various regimens. Adjuvant chemotherapy is usually a set course of
combi-nation chemotherapy that is given in a high dose to patients who have no
evidence of residual cancer after radiotherapy or surgery. The purpose is to
eliminate any residual cancer cells, typically with the intent to cure disease.
Neoadjuvant chemotherapy aims to
eradicate micrometastases or reduce inoperable disease in order to prepare
patients for surgery and/or radiotherapy. Induction
chemotherapy is usually a combination chemotherapy given in a high dose to
cause a remission. Maintenance
chemotherapy (consolidation chemotherapy) is a long-term and low-dose regimen
that is given to a patient in remission to maintain the remission by inhibiting
the growth of remaining cancer cells.
Related Topics