Chapter: Medical Physiology: Cerebral Blood Flow, Cerebrospinal Fluid, and Brain Metabolism
Cerebrospinal Fluid System
Cerebrospinal Fluid System
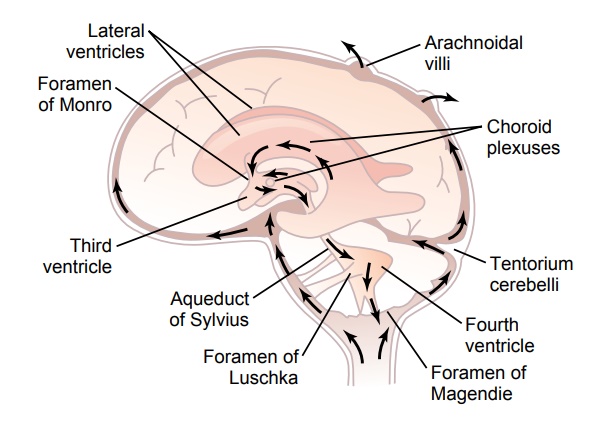
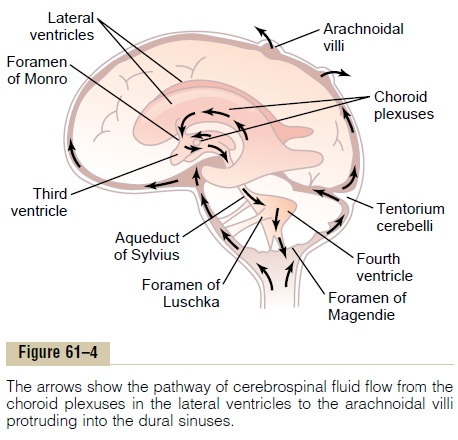
The entire cerebral cavity enclosing the brain and spinal cord has a capacity of about 1600 to 1700 milliliters; about 150 milliliters of this capacity is occupied by cere-brospinal fluid and the remainder by the brain and cord. This fluid, as shown in Figure 61–4, is present in the ven-tricles of the brain, in the cisterns around the outside of the brain, and in the subarachnoid space around both the brain and the spinal cord. All these chambers are con-nected with one another, and the pressure of the fluid is maintained at a surprisingly constant level.
Cushioning Function of the Cerebrospinal Fluid
A major function of the cerebrospinal fluid is to cushion the brain within its solid vault. The brain and the cere-brospinal fluid have about the same specific gravity (only about 4 per cent different), so that the brain simply floats in the fluid. Therefore, a blow to the head, if it is not too intense, moves the entire brain simulta-neously with the skull, causing no one portion of the brain to be momentarily contorted by the blow.
Contrecoup. When a blow to the head is extremely severe, it may not damage the brain on the side of the head where the blow is struck but on the opposite side. This phenomenon is known as “contrecoup,” and the reason for this effect is the following: When the blow is struck, the fluid on the struck side is so incompressible that as the skull moves, the fluid pushes the brain at the same time in unison with the skull. On the side oppo- site to the area that is struck, the sudden movement of the whole skull causes the skull to pull away from the brain momentarily because of the brain’s inertia, creat- ing for a split second a vacuum space in the cranial vault in the area opposite to the blow. Then, when the skull is no longer being accelerated by the blow, the vacuum suddenly collapses and the brain strikes the inner surface of the skull.
The poles and the inferior surfaces of the frontal and temporal lobes, where the brain comes into contact withbony protuberances in the base of the skull, are often the sites of injury and contusions (bruises) after a severe blow to the head, such as that experienced by a boxer. If the contusion occurs on the same side as the impact If the contusion occurs on the same side as the impact the contusion is a contrecoup injury.
Formation, Flow, and Absorption of Cerebrospinal Fluid
Cerebrospinal fluid is formed at a rate of about 500 mil-liliters each day, which is three to four times as much as the total volume of fluid in the entire cerebrospinal fluid system. About two thirds or more of this fluid originates as secretion from the choroid plexuses in the four ven-tricles, mainly in the two lateral ventricles. Additional small amounts of fluid are secreted by the ependymal surfaces of all the ventricles and by the arachnoidal membranes; and a small amount comes from the brain itself through the perivascular spaces that surround the blood vessels passing through the brain.
The arrows in Figure 61–4 show that the main chan-nels of fluid flow from the choroid plexuses and then through the cerebrospinal fluid system. The fluid secreted in the lateral ventricles passes first into the thirdventricle; then, after addition of minute amounts of fluid from the third ventricle, it flows downward along the aqueduct of Sylvius into the fourth ventricle, where still another minute amount of fluid is added. Finally, the fluid passes out of the fourth ventricle through three small openings, two lateral foramina of Luschka and a midline foramen of Magendie, entering the cisterna magna, a fluid space that lies behind the medulla and beneath the cerebellum.
The cisterna magna is continuous with the subarach- noid space that surrounds the entire brain and spinal cord. Almost all the cerebrospinal fluid then flows upward from the cisterna magna through the subarach- noid spaces surrounding the cerebrum. From here, the fluid flows into and through multiple arachnoidal villi that project into the large sagittal venous sinus and other venous sinuses of the cerebrum. Thus, any extra fluid empties into the venous blood through pores of these villi.
Secretion by the Choroid Plexus. Thechoroid plexus, asection of which is shown in Figure 61–5, is a cauliflower-like growth of blood vessels covered by a thin layer of epithelial cells. This plexus projects into (1 and 2) the temporal horn of each lateral ventricle, (3) the posterior portion of the third ventricle, and (4) the roof of the fourth ventricle.
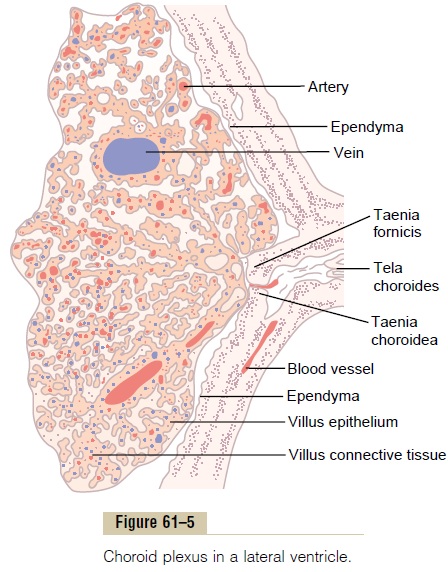
Secretion of fluid into the ventricles by the choroid plexus depends mainly on active transport of sodium ions through the epithelial cells lining the outside of the plexus.The sodium ions in turn pull along large amounts of chloride ions as well because the positive charge of the sodium ion attracts the chloride ion’s negative charge. The two of these together increase the quantity of osmotically active sodium chloride in the cere-brospinal fluid, which then causes almost immediate osmosis of water through the membrane, thus providing the fluid of the secretion.
Less important transport processes move small amounts of glucose into the cerebrospinal fluid and both potassium and bicarbonate ions out of the cerebrospinal fluid into the capillaries. Therefore, the resulting char-acteristics of the cerebrospinal fluid become the follow-ing: osmotic pressure, approximately equal to that of plasma; sodium ion concentration, also approximately equal to that of plasma; chloride ion, about 15 per cent greater than in plasma; potassium ion, approximately 40 per cent less; and glucose, about 30 per cent less.
Absorption of Cerebrospinal Fluid Through the Arachnoidal Villi.
The arachnoidal villi are microscopic fingerlike inward projections of the arachnoidal membrane through the walls and into the venous sinuses. Conglomerates of these villi form macroscopic structures called arach-noidal granulations that can be seen protruding into thesinuses. The endothelial cells covering the villi have been shown by electron microscopy to have vesicular passages directly through the bodies of the cells large enough to allow relatively free flow of (1) cerebrospinal fluid, (2) dissolved protein molecules, and (3) even par-ticles as large as red and white blood cells into the venous blood.
Perivascular Spaces and Cerebrospinal Fluid. The large arter-ies and veins of the brain lie on the surface of the brain but their ends penetrate inward, carrying with them a layer of pia mater, the membrane that covers the brain, as shown in Figure 61–6. The pia is only loosely adher-ent to the vessels, so that a space, the perivascular space, exists between it and each vessel. Therefore, perivascu-lar spaces follow both the arteries and the veins into the brain as far as the arterioles and venules go.
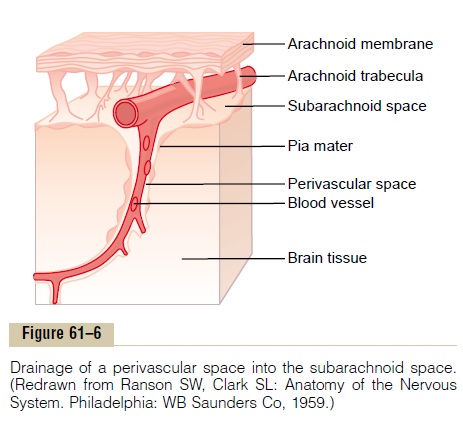
Lymphatic Function of the Perivascular Spaces. As is trueelsewhere in the body, a small amount of protein leaks out of the brain capillaries into the interstitial spaces of the brain. Because no true lymphatics are present in brain tissue, excess protein in the brain tissue leaves the tissue flowing with fluid through the perivascular spaces into the subarachnoid spaces. On reaching the sub-arachnoid spaces, the protein then flows with the cere-brospinal fluid, to be absorbed through the arachnoidalvilli into the large cerebral veins. Therefore, perivascular spaces, in effect, are a specialized lymphatic system for the brain.
In addition to transporting fluid and proteins, the perivascular spaces transport extraneous particulate matter out of the brain. For instance, whenever infec-tion occurs in the brain, dead white blood cells and other infectious debris are carried away through the perivascular spaces.
Cerebrospinal Fluid Pressure
The normal pressure in the cerebrospinal fluid system when one is lying in a horizontal position averages 130millimeters of water (10 mm Hg), although this may be as low as 65 millimeters of water or as high as 195 mil-limeters of water even in the normal healthy person.
Regulation of Cerebrospinal Fluid Pressure by the Arachnoidal Villi. The normal rate of cerebrospinal fluid formationremains very nearly constant, so that changes in fluid formation are seldom a factor in pressure control. Con-versely, the arachnoidal villi function like “valves” that allow cerebrospinal fluid and its contents to flow readily into the blood of the venous sinuses while not allowing blood to flow backward in the opposite direction. Nor-mally, this valve action of the villi allows cerebrospinal fluid to begin to flow into the blood when cerebrospinal fluid pressure is about 1.5 mm Hg greater than the pres-sure of the blood in the venous sinuses. Then, if the cere-brospinal fluid pressure rises still higher, the valves open more widely, so that under normal conditions, the cere-brospinal fluid pressure almost never rises more than a few millimeters of mercury higher than the pressure in the cerebral venous sinuses.
Conversely, in disease states, the villi sometimes become blocked by large particulate matter, by fibrosis, or by excesses of blood cells that have leaked into the cerebrospinal fluid in brain diseases. Such blockage can cause high cerebrospinal fluid pressure, as follows.
High Cerebrospinal Fluid Pressure in Pathological Conditions of the Brain. Often a largebrain tumorelevates the cere-brospinal fluid pressure by decreasing reabsorption of the cerebrospinal fluid back into the blood. As a result, the cerebrospinal fluid pressure can rise to as much as 500 millimeters of water (37 mm Hg) or about four times normal.
The cerebrospinal fluid pressure also rises consider-ably when hemorrhage or infection occurs in the cranial vault. In both these conditions, large numbers of red and/or white blood cells suddenly appear in the cere-brospinal fluid, and they can cause serious blockage of the small absorption channels through the arachnoidal villi. This also sometimes elevates the cerebrospinal fluid pressure to 400 to 600 millimeters of water (about four times normal).
Some babies are born with high cerebrospinal fluid pressure. This is often caused by abnormally high resist-ance to fluid reabsorption through the arachnoidal villi, resulting either from too few arachnoidal villi or from villi with abnormal absorptive properties. This is dis-cussed later in connection with hydrocephalus.
Measurement of Cerebrospinal Fluid Pressure. The usual pro-cedure for measuring cerebrospinal fluid pressure is very simple and is the following: First, the person lies exactly horizontally on his or her side so that the fluid pressure in the spinal canal is equal to the pressure in the cranial vault. A spinal needle is then inserted into the lumbar spinal canal below the lower end of the cord, and the needle is connected to a vertical glass tube that is open to the air at its top. The spinal fluid is allowed to rise in the tube as high as it will. If it rises to a level 136 millimeters above the level of the needle, the pres-sure is said to be 136 millimeters of water pressure or, dividing this by 13.6, which is the specific gravity of mercury, about 10 mm Hg pressure.
High Cerebrospinal Fluid Pressure Causes Edema of the Optic Disc—Papilledema. Anatomically, the dura of the brainextends as a sheath around the optic nerve and then connects with the sclera of the eye. When the pressure rises in the cerebrospinal fluid system, it also rises inside the optic nerve sheath. The retinal artery and vein pierce this sheath a few millimeters behind the eye and then pass along with the optic nerve fibers into the eye itself. Therefore, (1) high cerebrospinal fluid pressure pushes fluid first into the optic nerve sheath and then along the spaces between the optic nerve fibers to the interior of the eyeball; (2) the high pressure decreases outward fluid flow in the optic nerves, causing accumu-lation of excess fluid in the optic disc at the center of the retina; and (3) the pressure in the sheath also impedes flow of blood in the retinal vein, thereby increasing the retinal capillary pressure throughout the eye, which results in still more retinal edema.
The tissues of the optic disc are much more distensi-ble than those of the remainder of the retina, so that the disc becomes far more edematous than the remainder of the retina and swells into the cavity of the eye. The swelling of the disc can be observed with an ophthal-moscope and is calledpapilledema. Neurologists can estimate the cerebrospinal fluid pressure by assessing the extent to which the edematous optic disc protrudes into the eyeball.
Obstruction to Flow of Cerebrospinal Fluid Can Cause Hydrocephalus
“Hydrocephalus” means excess water in the cranial vault. This condition is frequently divided into commu-nicating hydrocephalus andnoncommunicating hydro-cephalus. In communicating hydrocephalus fluid flowsreadily from the ventricular system into the subarach-noid space, whereas in noncommunicating hydro-cephalus fluid flow out of one or more of the ventricles is blocked.
Usually the noncommunicating type of hydro-cephalus is caused by a block in the aqueduct of Sylvius, resulting from atresia (closure) before birth in many babies or from blockage by a brain tumor at any age. As fluid is formed by the choroid plexuses in the two lateral and the third ventricles, the volumes of these three ventricles increase greatly. This flattens the brain into a thin shell against the skull. In neonates, the increased pressure also causes the whole head to swell because the skull bones have not yet fused.
The communicating type of hydrocephalus is usually caused by blockage of fluid flow in the subarachnoid spaces around the basal regions of the brain or by block-age of the arachnoidal villi where the fluid is normally absorbed into the venous sinuses. Fluid therefore col-lects both on the outside of the brain and to a lesser extent inside the ventricles. This will also cause the head to swell tremendously if it occurs in infancy when the skull is still pliable and can be stretched, and it can damage the brain at any age. A therapy for many types of hydrocephalus is surgical placement of a silicone tube shunt all the way from one of the brain ventricles to the peritoneal cavity where the excess fluid can be absorbed into the blood.
Blood–Cerebrospinal Fluid and Blood-Brain Barriers
It has already been pointed out that the concentrations of several important constituents of cerebrospinal fluid are not the same as in extracellular fluid elsewhere in the body. Furthermore, many large molecular sub-stances hardly pass at all from the blood into the cere-brospinal fluid or into the interstitial fluids of the brain, even though these same substances pass readily into the usual interstitial fluids of the body. Therefore, it is said that barriers, called the blood–cerebrospinal fluidbarrier and the blood-brain barrier, exist between theblood and the cerebrospinal fluid and brain fluid, respectively.
Barriers exist both at the choroid plexus and at the tissue capillary membranes in essentially all areas of the brain parenchyma except in some areas of the hypothal-amus, pineal gland, and area postrema, where substancesdiffuse with greater ease into the tissue spaces. The ease of diffusion in these areas is important because they have sensory receptors that respond to specific changes in the body fluids, such as changes in osmolality and in glucose concentration, as well as receptors for peptide hormones that regulate thirst, such as angiotensin II. The blood-brain barrier also has specific carrier mole-cules that facilitate transport of hormones, such as leptin, from the blood into the hypothalamus where they bind to specific receptors that control other func-tions such as appetite and sympathetic nervous system activity.
In general, the blood–cerebrospinal fluid and blood-brain barriers are highly permeable to water, carbon dioxide, oxygen, and most lipid-soluble substances such as alcohol and anesthetics; slightly permeable to electrolytes such as sodium, chloride, and potassium; and almost totally impermeable to plasma proteins and most non–lipid-soluble large organic molecules. There-fore, the blood–cerebrospinal fluid and blood-brain bar-riers often make it impossible to achieve effective concentrations of therapeutic drugs, such as protein antibodies and non–lipid-soluble drugs, in the cere-brospinal fluid or parenchyma of the brain.
The cause of the low permeability of the blood– cerebrospinal fluid and blood-brain barriers is the manner in which the endothelial cells of the brain tissue capillaries are joined to one another. They are joined by so-called tight junctions. That is, the membranes of the adjacent endothelial cells are tightly fused rather than having large slit-pores between them, as is the case for most other capillaries of the body.
Brain Edema
One of the most serious complications of abnormal cerebral fluid dynamics is the development of brainedema. Because the brain is encased in a solid cranialvault, accumulation of extra edema fluid compresses the blood vessels, often causing seriously decreased blood flow and destruction of brain tissue.
The usual cause of brain edema is either greatly increased capillary pressure or damage to the capillary wall that makes the wall leaky to fluid. A very common cause is a serious blow to the head, leading to brain con-cussion, in which the brain tissues and capillaries aretraumatized so that capillary fluid leaks into the trau-matized tissues.
Once brain edema begins, it often initiates two vicious circles because of the following positive feedbacks:
(1) Edema compresses the vasculature. This in turn decreases blood flow and causes brain ischemia. The ischemia in turn causes arteriolar dilation with still further increase in capillary pressure.The increased cap-illary pressure then causes more edema fluid, so that the edema becomes progressively worse. (2) The decreased cerebral blood flow also decreases oxygen delivery. This increases the permeability of the capillaries, allowing still more fluid leakage. It also turns off the sodium pumps of the neuronal tissue cells, thus allowing these cells to swell in addition.
Once these two vicious circles have begun, heroic measures must be used to prevent total destruction of the brain. One such measure is to infuse intravenously a concentrated osmotic substance, such as a very con-centrated mannitol solution. This pulls fluid by osmosis from the brain tissue and breaks up the vicious circles. Another procedure is to remove fluid quickly from the lateral ventricles of the brain by means of ventricular needle puncture, thereby relieving the intracerebral pressure.
Related Topics