Acids, Bases and Salts | Chemistry | Science - Answer the following questions | 9th Science : Chemistry : Acids, Bases and Salts
Chapter: 9th Science : Chemistry : Acids, Bases and Salts
Answer the following questions
CHEMISTRY
ACIDS,
BASES AND SALTS
TEXT BOOK EXERCISES
II. Answer briefly:
1. Classify the various types of
Acids based on their sources.
Answer: The
acids are classified based on their sources and organic and inorganic acids.
Organic acids - acids present in plants and animals.
Inorganic
acids - acids prepared from rocks and minerals.
2. Write any four uses of acids.
Answer:
(i)
Sulphuric acid is called King of Chemicals because it is used in the
preparation of many other compounds. It is used in car batteries also.
(ii)
Hydrochloric acid is used as a cleansing agent in toilets.
(iii)
Citric acid is used in the preparation of effervescent salts and as a food
preservative.
(iv)
Nitric acid is used in the manufacture of fertilizers, dyes, paints and drugs.
(v)
Oxalic acid is used to clean iron and manganese deposits from quartz crystals.
It is also used as bleach for wood and removing black stains.
(vi)
Carbonic acid is used in aerated drinks.
(vii)
Tartaric acid is a constituent of baking powder.
3. Give the significance of pH of
soil in agriculture.
Answer: In
agriculture, the pH of soil is very important. Citrus fruits require slightly
alkaline soil, while rice requires acidic soil and sugarcane requires neutral
soil.
4. What are the various uses of
Aquaregia.
Answer: (i) It
is used chiefly to dissolve metals such as gold and platinum.
(ii)
It is used for cleaning and refining gold.
5. What are the uses of Plaster
of Paris?
Answer: (i) It
is used for plastering bones.
(ii)
It is used for making casts for statues.
6. Two acids ‘A’ and ‘B’ are
given. Acid A gives one hydrogen ion per molecule of the acid in solution. Acid
B gives two hydrogen ions per molecule of the acid in solution.
(i) Find out acid A and acid B.
(ii) Which acid is called the
King of Chemicals?
Answer:
(i)
Acid A
- HCl- Hydrochloric acid. Acid B
- H2SO4 - Sulphuric acid.
(ii)
Sulphuric acid - H2SO4.
7. Define aquaregia.
Answer: It is
the mixture of hydrochloric acid and nitric acid prepared optimally in a molar
ratio of 3:1.
8. Correct the mistakes:
(a) Washing soda is used for
making cakes and bread soft, spongy.
(b) Calcium sulphate hemihydrate
is used in textile industry for bleaching cloths.
Answer: (a)
Baking soda (Sodium bicarbonate – NaHCO3 is used for making cakes
and bread soft spongy, (or) Washing soda is used for softening hard water.
(b)
Calcium sulphate hemihydrate (CaSO4, 1/2 H2,O)is used for
plastering bones (or) Bleaching powder (Calcium oxy Chloride - CaOC12)
is used in textile industry.
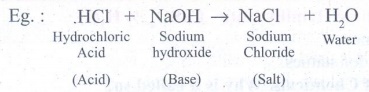
9. What is neutralization
reaction? Give an example.
Answer: Neutralization
reaction is a reaction in which an acid reacts with a base to form salt and
water and H+ ion and OH− ion combines to generate water.
The neutralization of a strong acid and strong base has a pH equal 7.
III. Answer in detail:
1. Differentiate hydrate and
anhydrous salts with examples.
Answer:
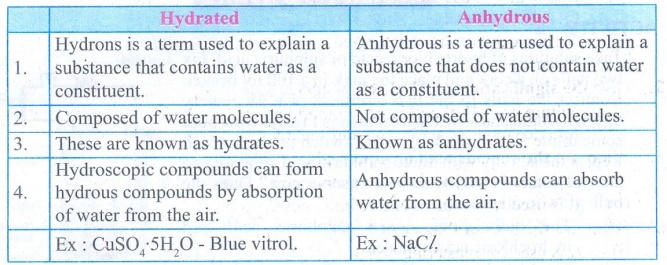
Hydrated
1.
Hydrons is a term used to explain a substance that contains water as a
constituent.
2.
Composed of water molecules.
3.
These are known as hydrates
4.
Hydroscopic compounds can form hydrous compounds by absorption of water from the
air
Ex:
CUSO4.5H2O - Blue vitrol.
Anhydrous
1.
Anhydrous is a term used to explain a substance that does not contain water as
a constituent.
2.
Not composed of water molecules.
3.
Known as anhydrates
4.
Anhydrous compounds can absorb water from the air.
Ex:
NaCl.
2. Give the tests to identify
Acids and Bases.
Answer:
(i)
Acids turn blue litmus red, bases turn red litmus blue.
(ii)
In acid, phenolphthalein is colourless. In base Phenolphthalein is pink in
colour.
(iii)
In acid, methyl orange is pink. In base, methyl orange is yellow.
3. Write any four uses of bases.
(i)
Sodium hydroxide is used in the manufacture of soap.
(ii)
Calcium hydroxide is used in white washing of building.
(iii)
Magnesium hydroxide is used as a medicine for stomach disorder.
(iv)Ammonium
hydroxide is used to remove grease stains from cloth.
4. Write any five uses of salts.
Answer:
Common Salt (NaCl):
It
is used in our daily food and used as a preservative.
Washing Soda
(Sodium Carbonate - Na2CO3):
(i)
It is used in softening hard water.
(ii)
It is used in glass, soap and paper industries.
Baking Soda (Sodium
bicarbonate -NaHCO3):
(i)
It is used in making of baking powder which is a mixture of baking soda and
tartaric acid.
(ii)
It is used in soda-acid fire extinguishers.
(iii)
Baking powder is used to make cakes and bread, soft and spongy.
(iv)
It neutralizes excess acid in the stomach and provides relief.
Bleaching powder
(Calcium Oxychloride - CaOCl2):
(i)
It is used as disinfectant.
(ii)
It is used in textile industry for bleaching cotton and linen.
Plaster of Paris
(Calcium Sulphate Hemihydrate - CaSO4.1/2 H2O):
(i)
It is used for plastering bones
(ii)
It is used for making casts for statues.
5. Sulphuric acid is called King
of Chemicals. Why is it called so?
Answer:
Sulphuric acid is called King of Chemicals because it is used in the
preparation of many other compounds.
Intext Activities
ACTIVITY - 2
Take solutions of hydrochloric
acid or sulphuric acid. Fix two nails on a cork and place the cork in a 100 ml
beaker. Connect the nails to the two terminals of a 6V battery through a bulb
and a switch as shown in Figure. Now pour some dilute HCl in the beaker and switch on the current. Repeat the activity with
dilute sulphuric acid, glucose and alcohol solutions. What do you observe now?
Does the bulb glow in all cases?
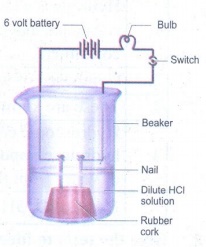
Answer: (i) The
bulb glows when sulphuric acid and hydrochloric acid are used.
(ii)
The bulb does not glow when the activity is done with alcohol and glucose
solution.
ACTIVITY - 3
Collect the following samples
from the science laboratory — Hydrochloric acid, Sulphuric acid and Nitric
acid, Sodium hydroxide, Potassium hydroxide. Take 2 ml of each solution in a
test tube and test with a litmus paper and indicators phenolphthalein and
Methyl orange. Tabulate your observations.
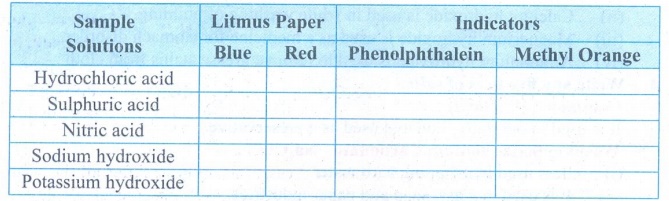
Answer:

ACTIVITY - 4
Fill in the blanks in the
following table based on the concept of water of crystallisation.
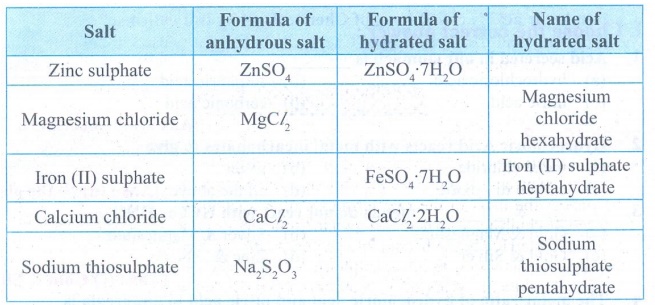
Answer:
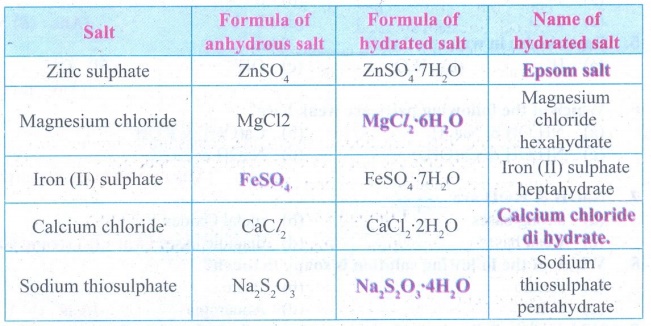
ACTIVITY – 5
Boil about 100 ml of ground water
in a vessel to dryness. After all the water get evaporated observe the inner
wall of the vessel. Can you observe any deposits? This is the deposit of
dissolved salts present in water.
Answer: This is
the deposit of dissolved salts present in water.
Related Topics