Chapter: Physics : Advanced Engineering Materials Metallic Glasses
Advanced Engineering Materials Metallic Glasses
ADVANCED ENGINEERING MATERIALS METALLIC
GLASSES
1 Introduction
2. Metallic glasses
2.1
Methods of preparation
2.2
Preparation of metallic glasses
2.3
Types of metallic glasses
2.4
Properties of metallic glasses
2.5
Applications of metallic glasses
3
Shape memory alloys
3.1
Shape memory alloys
3.2
Types of shape memory alloys
3.3
Characteristics of SMA
3.4
Commercial shape memory alloys
3.5
Advantages of shape memory alloys
3.6
Disadvantages of shape memory alloys
3.7
Applications of shape memory alloys
4
Nanotechnology
4.1
Nano materials
4.2
Comparison of different objects
4.3
Classification of nanomaterials
4.4
Top-down and bottom-up process
5
Synthesis techniques
5.1
Pulsed laser deposition
5.2
Chemical vapor deposition
6
Discuss the properties of nanophase materials
6.1
Physical properties
6.2
Magnetic properties
6.3
Mechanical properties
7
Applications of nanophase materials
8 Non-linear materials and bio-materials
8.1
Birefringence and Kerr effect
8.2
Non-linear properties and second harmonic generation
8.3
Non linear properties
8.4
Second harmonic generation
8.5
Biomaterials with their properties and applications
8.6
Classification of biomaterials
8.7
Applications
8.8
Ceramic
1 INTRODUCTION
New engineering
materials such as metallic glasses, shape memory alloys etc. are the advanced
materials, which are the integral part of our life. Both scientists and
technologists are searching for new materials, which can be used for high
technology research as well as applications.
In this chapter, we are
going to discuss the new engineering materials like metallic glasses, shpe
memory alloys, etc., along with their properties and its wide range of
applications.
2 METALLIC GLASSES
The Metallic glasses are materials which
have the properties of both metals and glasses.
Metallic glass = Amorphous metal
In general, metallic glasses are strong, ductile,
malleable, opaque and brittle. They also have good magnetic properties and high
corrosion resistance.
2.1METHODS
OF PREPARATION Principle
The principle used in
making metallic glasses is extreme rapid cooling of the molten alloy. The
technique is called as rapid quenching.
The cooled molten
alloys are fed into highly conducting massive rollers at high speeds to give
ribbons of metallic glasses.
2.1PREPARATION
OF METALLIC GLASSES
Principle
The principle used in making metallic glasses is
extreme rapid cooling of the molten metal alloy. This technique is called as
rapid quenching.
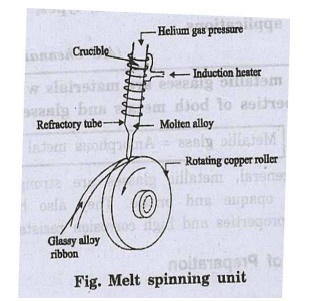
Melt spinning system
A melt spinner consists
of a copper roller over which a refractory tube with fine nozzle is placed. The
refractory tube is provided with induction heater as shown in fig.
The metal alloy is
melted by induction heating under inert gas atmosphere (helium or argon). The
properly super heated molten alloy is ejected through the fine nozzle at the
bottom of the refractory tube.
The molten alloy falls on the copper
roller which is rotated at high speed. Thus, the alloy is suddenly cooled to
form metallic glass. In this method a continuous ribbon of metallic glass can
be obtained.
2.3 TYPES OF METALLIC
GLASSES.
Metallic glasses are classified into two types:
(i)Metal –Metal metallic glasses
They are combination of metals
Metals Metals
Examples: Nickel (Ni) - Niobium (Nb)
Magnesium
(Mg) - Zinc
(Zn)
Copper
(Cu) - Zirconium (Zr)
(ii)
Metal –Metalloid metallic glasses
These
are combinations of metals and metalloids.
Examples: Metals Metalloids
Fe, Co, Ni - B, Si, C, P
2.4 PROPERTIES
OF METALLIC GLASSES
Structural properties
1. They
do not have any crystal defects such as grain boundaries, dislocation etc.
2. Metallic
glasses have tetrahedral close packing (TCP).
Mechanical properties
1. Metallic
glasses have extremely high strength, due to the absence of point defects and
dislocation.
2. They
have high elasticity.
3. They
are highly ductile.
4. Metallic
glasses are not work-harden but they are work –soften. (work harnening is a
process of hardening a material by compressing it).
Electrical properties
1. Electrical
resistivity of metallic glasses is high and it does not vary much with
temperature.
2. Due
to high resistivity, the eddy current loss is very small.
3. The
temperature coefficient is zero or negative.
Magnetic properties
1. Metallic
glasses have both soft and hard magnetic properties.
2. They
are magnetically soft due to their maximum permeabilities and thus they can be
magnetised and demagnetized very easily.
3. They
exhibit high saturation magnetisation.
4. They
have less core losses.
5. Most
magnetically soft metallic glasses have very narrow hysteresis loop with same
crystal composition. This is shown in fig.
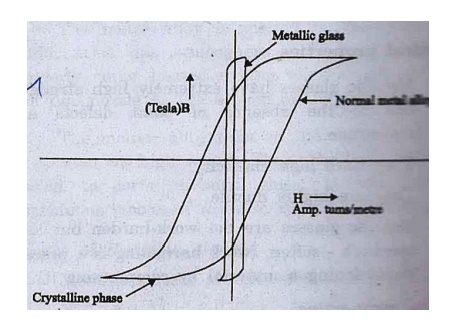
Fig.
Hysteresis loop of iron based alloy in crystalline and metallic glassy phase.
Chemical properties
1. They
are highly resistant to corrosion due to random ordering.
2. They
are highly reactive and stable.
3. They
can act as a catalyst. The amorphous state is more active than the crystalline
state from the catalytic point of view.
2.5APPLICATIONS
OF METALLIC GLASSES
Metallic glasses also called as met glasses have
found wide applications in different fields.
Structural application
1. They
posses high physical and tensile strength. They are superior to common steels
and thus they are very useful as reinforcing elements in concrete, plastic and
rubber.
2. Strong
ribbons of metallic glasses are used for simple filament winding to reinforce
pressure vessels and to construct large fly wheels for energy storage.
3. Due
to their good strength, high ductility, rollability and good corrosion
resistance, they are used to make razor blades and different kinds of springs.
Electrical and Electronics
1. Since
metallic glasses have soft magnetic properties, they are used in tape recorder
heads, cores of high-power transformers and magnetic shields.
2. They
use of metallic glasses in motors can reduce core loss very much when compared
with conventional crystalline magnets.
3. Superconducting
metallic glasses are used to produce high magnetic fields and magnetic
levitation effect.
4. Since
metallic glasses have high electrical resistance, they are used to make
accurate standard resistance, computer memories and magneto resistance sensors.
Metallic glasses as transformer core
material
5. Metallic
glasses have excellent magnetic properties. When they are used as transformer
core, they give maximum magnetic flux linkage between primary and secondary
coils and thus reduce flux leakage losses.
In view of their features like small thickness,
smaller area, light weight, high resistivity, soft magnetic property and
negligible hysteresis and eddy current loss, metallic glasses are considered as
suitable core materials in different frequency transformers.
Nuclear reactor engineering
1.The magnetic
properties of metallic glasses are not affected by irradiation and so they are
useful in preparing containers for nuclear waste disposal and magnets for
fusion reactors.
2.Chromium and
phosphorous based (iron chromium, phosphorous-carbon alloys) metallic glasses
have high corrosion resistances and so they are used in iner surfaces of
reactor vessels, etc.
Bio-medical Industries
1. Due
to their high resistance to corrosion, metallic glasses are ideal materials for
making surgical instruments.
2. They
are used as prosthetic materials for implantation in human body.
2 SHAPE
MEMORY ALLOYS
3.1 SHAPE MEMORY ALLOYS
A group of metallic
alloys which shows the ability to return to their original shape or size (i.e.,
alloy appears to have memory) when they
are subjected to heating or cooling are called shape memory alloys.
Phase of shape memory alloys
Martensite and
austenite are two solid phases in SMA as shown in fig.
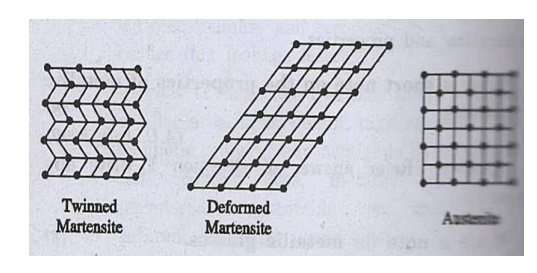
Fig. Phases of SMA
Martensite is
relatively soft and it is easily deformable phase which exists at low
temperature (monoclinic) (fig.)
(i)
Austenite is a phase that occurs at high
temperature having a crystal structure and high degree of symmetry (cubic)
(fig.).
3.2TYPES
OF SHAPE MEMORY ALLOYS
There are two types of shape memory alloys
(i)
One-way shape memory alloy
(ii)
Two-way shape memory alloy
A material which
exhibits shape memory effect only upon heating is known as one-way shape
memory. A material which shows a shape memory effect during both heating and
cooling is called two-way shape memory.
Examples of shape memory alloys
Generally, shape memory
alloys are intermetallic compounds having super lattice structures and
metallic-ionic-covalent characteristics. Thus, they have the properties of both
metals and ceramics.
Ni –Ti alloy (Nitinol)
Cu –Al –Ni alloy
Cu –Zn –Al alloy
Au –Cd alloy
Ni –Mn –Ga and Fe based
alloys
3.3CHARACTERISTICS
OF SMAS
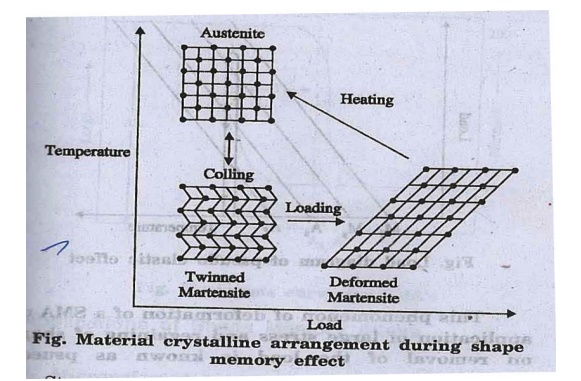
1. Shape memory
effect
The change of shape of
a material at low temperature by loading and regaining of original shape by
heating it, is known as shape memory effect.
The shape memory effect
occurs in alloys due to the change in their crystalline structure with the
change in temperature and stress.
While loading, twinned
martensite becomes deformed martensite at low temperature.
On heating, deformed
martensite becomes austenite (shape recovery) and upon cooling it gets
transformed to twinned martensite (fig.).
2.SMAs exhibit changes in electrical resistance,
volume and length during the transformation with temperature.
3.The mechanism involved in SMA is reversible
(austenite to martensite and vice versa.)
4. Stress
and temperature have a great influence on martensite transformation.
5. Pseudo
elasticity
Pseudo –elasticity
occurs in shape memory alloys when it is completely in austenite phase
(temperature is greater than Af austenite finish
temperature).
Unlike the shape memory
effect, Pseudo-elasticity occurs due to stress induced phase transformation
without a change in temperature. The load on the shape memory alloy changes
austenite phase into martensite (Fig.).
As soon as the loading decreases the martensite
begins to transform to austenite.
This phenomenon of
deformation of a SMA on application of large stress and regaining of shape on
removal of the load is known as pseudo elasticity.
This pseudo elasticity is also known as super
elasticity
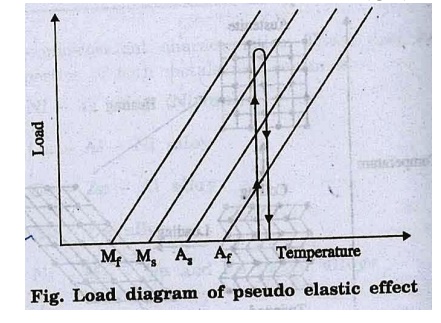
6. Hysteresis
The temperature range
for the martensite to austenite transformation which takes place upon heating
is somewhat higher than that for the reverse transformation upon cooling.
The difference between
the transition temperature upon heating and cooling is called hysteresis. The
hysteresis curve for SMAs is shown in fig.
The
difiference of temperature is found to be 20-30oC,
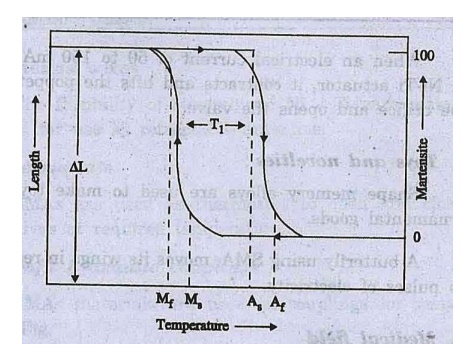
3.4 COMMERCIAL SHAPE
MEMORY ALLOYS
The only two alloy systems that have achieved any
level of commercial exploitation are,
(i)
Ni-Ti alloys, and
(ii)
Copper base alloys.
Properties
of the two systems are quite different.
1. Nickel-Titanium
Alloys
The basis of the
Nickel-Titanium alloy is the binary, equi-atomic inter-metallic compound of
Ti-Ni. The inter-metallic compound is extraordinary because it has moderate
solubility range for excess Nickel or Titanium, as well as most other metallic
elements. This solubility allows alloying with many of the elements to modify
both the mechanical properties and the transformation properties of the system.
Excess Nickel strongly depresses the transformation temperature and increases
the yield strength of the austenite. The contaminants such as Oxygen and Carbon
shift the transformation temperature and degrade the mechanical properties.
Therefore, it is also desirable to minimize the amount of such elements.
Properties:
(i)
The Ni-Ti alloys have greater shape
memory strain upto 8.5% tend to be much more thermally stable.
(ii)
They have excellent corrosion resistance
and susceptibility, and have much higher ductility.
(iii)
Machining by turning or milling is very
difficult except with special tools.
(iv)
Welding, brazing or soldering the alloys
is generally difficult.
(v)
The material do respond well to abrasive
removal such as grinding, and shearing.
(vi)
Punching can be done if thicknesses are
kept small.
3.5 ADVANTAGES OF SHAPE
MEMORY ALLOYS
They are simple, compact and high safe.
They have good bio –compatibility.
They have diverse applications and offer clean, silent and spark-free
working condition
They have good mechanical properties and are strong corrosion-resistant.
3.6DISADVANTAGES
OF SHAPE MEMORY ALLOYS
They have poor fatigue properties.
They are expensive.
They have low energy efficiency.
3.7 APPLICATIONS
OF SHAPE MEMORY ALLOYS
1. Microvalve (Actuators)
One of the most common
applications of SMAs is mocrovalves. Fig. shows a microvalve made of Ni –Ti
alloy actuator. Actuator is a microsensor that can trigger the operation of a
device. The electrical signal initiates an action.
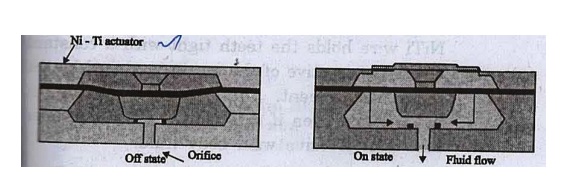
Fig. Schematic of microvalves that open
and close according to temperature
When an electrical
current of 50 to 150 mA flows in Ni-Ti actuator, it contracts and lifts the
poppet from the orifice and opens the valve.
2. Toys and novelties
Shape
memory alloys are used to make toys and ornamental goods.
A
butterfly using SMA. Moves its wings in response to pulses of electricity.
3. Medical field Blood
clot filters
(i)
Blood clot filters are SMAs, properly
shaped and inserted in veins to stop the passing blood clots.
When the SMA is in contact with the clot at a lower
temperature, it expands and stops the clot and blood passes through the veins.
(ii)
They are used in artificial hearts.
(iii)
Orthodontic applications
NiTi wire holds the
teeth tight with a constant stress irrespective of the strain produced by the
teeth movement. It resists permanent deformation even if it is bent. NiTi is
non-toxic and non-corrosive with body fluid.
(iv)
SMAs (NiTi) are used to make eye glass
frames and medical tools. Sun-glasses made from superelastic Ni-Ti frames
provide good comfort and durability.
4. Antenna
wires
The
flexibility of superelastic Ni –Ti wire makes it ideal for use as retractable
antennas.
5. Thermostats
SMAs
are used as thermostat to open and close the valves at required temperature.
6. Cryofit hydraulic couplings
SMAs
materials are used as couplings for metal pipes
7. Springs, shock absorbers, and valves
Due to the excellent
elastic property of the SMAs, springs can be made which have varied industrial
applications. Some of them are listed here.
Engine micro valves
Medical stents (Stents
are internal inplant supports provided for body organs)
Firesafety valves and
Aerospace latching
mechanisms
8. Stepping motors
Digital
SMA stepping motors are used for robotic control.
9. Titanium-aluminium
shape memory alloys offer excellent strength with less weight and dominate in
the aircraft industry. They are high temperature SMAs, for possible use in
aircraft engines and other high temperature environments.
4
NANOTECHNOLOGY
4.1 NANO MATERIALS
Nanoparticles are the
particles that have three dimensional nanoscale, the particle is between 1 and
100 nm in each spatial dimension. A nanometer is a unit of measure equal to
one-billionth of a meter, or three to five atoms across.
Nanotechnology is the design, fabrication and use of
nanostructured systems, and the growing, assembling of such systems either
mechanically, chemically or biologically to form nanoscale architectures,
systems and devices.
4.2COMPARISON
OF DIFFERENT OBJECTS
1. Diameter of sun - 1,393,000km
2. Diameter of earth - 1,28,000km
3. Height of Himalaya mountain - 8,848km
4. Height of man - 1.65km
5. Virus - 20-250nm
6. Cadmium sulphide nanoparticle - 1-10nm
4.3CLASSIFICATION
OF NANOMATERIALS
1. Clusters
A collection of atoms or reactive molecules up to
about 50 units.
2. Colloid
A stable liquid phase containing particles in 1 to
1000 nm range. A colloidal particle is
one such 1 to
1000 nm sized particle.
3. Nanoparticle
A solid particle in the 1 to 100 nm range that could
be non-crystalline, an aggregate of crystallites, or a single crystallite.
4. Nanocrystal
A solid particle that is a single crystal in the
nanometer size.
5. Nanostructured or Nanoscale Material
Any solid materials has a nanometer dimension.
Three dimensions --- > Particles
Two dimensions --- > Thin films
One dimension --- > Thin wire
6. Quantum Dots
A particle that exhibits a size quantization effect
in at least one dimension.
4.4 TOP-DOWN AND
BOTTOM-UP PROCESSS
1. Top-down Process
In this processes, bulk
materials are broken into nano sized particles as shown in
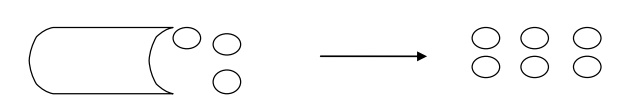
In to-down processes,
the building of nanostructures starting with small components like atoms and
molecules that are removed from a bulk material so as to obtain desired
microstructure.
2. Bottom-up Processes
In this processes, nano
phase materials are produced by building of atom by atom as shown in.
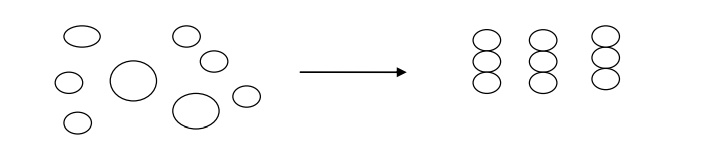
This processes building
larger objects from smaller buildings blocks. Nanotechnology seeks to use atoms
and molecules as those building blocks. This is the opposite of the top-down
approach. Instead of taking material away to make structures, the bottom-up
approach selectively adds atoms to create structures.
5 SYNTHESIS TECHNIQUES
Nano materials are newly developed materials with
grain size at the nanometre range (10-9m)
i.e., in the order of 1 –100 nm. The particle size
in a nano material is in the order of nm.
5.1 PULSED LASER
DEPOSITION
Priniciple
The laser pulse of high
intensity and energy is used to evaporate carbon from graphite. These
evaporated carbon atoms are condensed to from nanotubes.
Description
The experimental
arrangement of pulsed laser4 deposition is shown in fig. A quartz tube which
contains a graphite target is kept inside a high temperature muffle furnace.
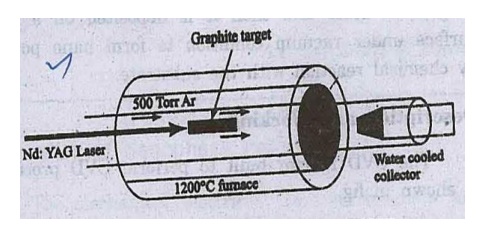
Fig.
Pulsed Laser Deposition CNT
This quartz tube is
filled with argon gas and it is heated to 1473 K. A water cooled copper
collector is fitted at the other end of the tube. The target material graphite
contains small amount of nickel and cobalt as a catalyst to nucleate the
formation of nanotubes.
Working
When an intense pulse
of laser beam is incident on the target, it evaporates the carbon from the
graphite. The evaporated carbon atoms are swept from the higher
temperature argon gas to the colder copper collector.
When the carbon atoms reach the colder copper
collector, they condense into nanotubes.
5.2CHEMICAL
VAPOUR DEPOSITION
The deposition of nano
films from gaseous phase by chemical reaction on high temperature is known as chemical vapour deposition.
This
method is used to prepare nano-powder.
Principle
In this technique,
initially the material is heated to gaseous state and then it is deposited on a
solid surface under vacuum condition to form nano powder by chemical reaction
with the substrate.
Description and Working
The
CVD reactor built to perform CVD processes is shown in fig.
Chemical vapour
deposition (CVD) involves the flow of a gas with diffused reactants (substances
to be deposited in the vapour) over a hot substrate surface. The gas that
carries the reactants is called the carrier gas.
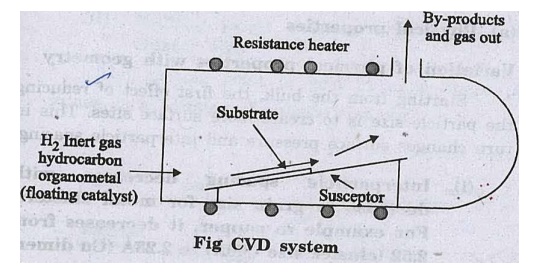
While the gas flows
over the hot solid surface, the heat energy increases chemical reactions of the
reactants that form film during and after the reactions.
The byproduct of the
chemical reactions are then removed. The thin film of desired composition can
thus be formed over the surface of the substrate.
6 PROPERTIES OF NANOPHASE MATERIALS.
Properties of Nanophase Particles
The mechanical,
electrical, chemical, magnetic and structural properties of nanophase materials
change with the reduction in the particle size of the material.
6.1 PHYSICAL PROPERTIES
Variation of physical properties with
geometry
Starting from the bulk,
the first effect of reducing the particle size is to create more surface sites.
This in turn changes surface pressure and interparticle spacing.
(i)
Interparticle spacing decreases
with decrease in grain size for metal clusters.
For
example in copper, it decrease from 2.52 (cluster size –50A) to 2.23A (Cu
dimer) fig.
The change in inter
particle spacing and large surface to the volume ratio in particles have a
combined effect on material properties. Therefore, the nanophase materials have
very high strength and super hardness.
Because of the cluster
of grains, the nano phase materials are mostly free from dislocations and
stronger than conventional metals.
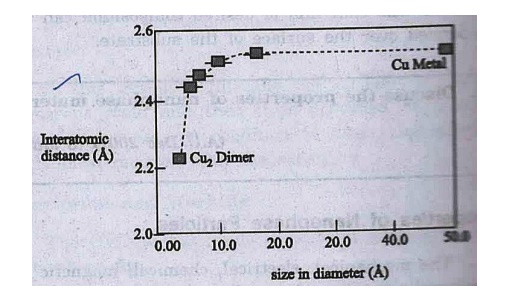
Fig.
Interatomic distance in Cun as a function of grain size.
(ii)
Melting point reduces with decrease
in cluster size.
The melting point of
gold in nano phase (Aun) varies as a function of particle size
(fig.)
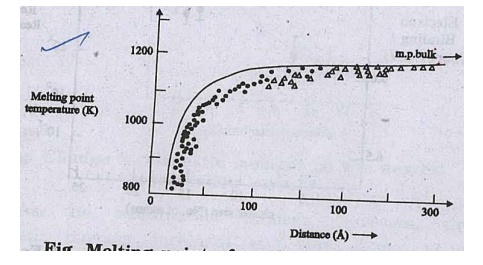
Fig.
Melting point of small Aun particles as a function of size
The melting point decreases
from 1200 K to 800 K when the particle size decreases from 300 A to 20 A.
(iii)
Ionisation potential changes with
cluster size of the nanograins.
The electronic bands in
metals become narrower when the size is reduced from bulk which changes the value
of ionization potential.
Fig. shows the
ionization potential and reactivity of Fen clusters as a function of
size. Ionisation potentials are higher at small sizes than that for the bulk
and show marked fluctuations as a function of size.
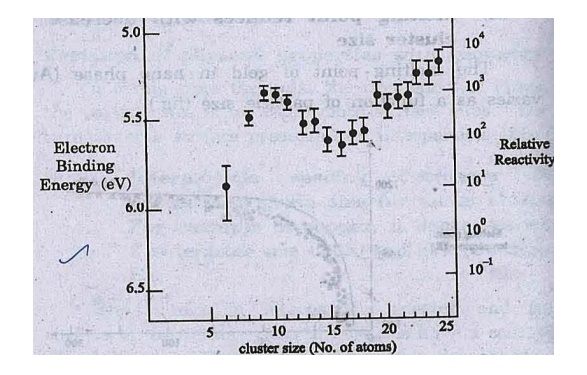
Fig. Ionisation
potential and reactivity of Fen clusters as a function of size (iv) The large
surface to volume ratio, the variations in geometry and the electronic structure
have a strong effect on catalytic properties.
As an example, the
reactivity of small clusters is found to vary by higher orders of magnitude
when the cluster size is changed by only a few atoms.
6.2 MAGNETIC PROPERTIES
Nanoparticles
of non-magnetic solids also exhibit totally new type of magnetic properties.
(i)
Bulk magnetic moment increases with
decrease in co-ordination number
The change in magnetic
moment on the nearest coordination number is shown in fig.-0
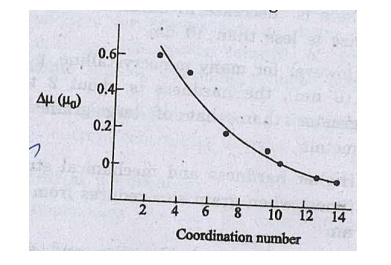
Fig.
Change in magnetic moment on the nearest coordination number
As the coordination
number decreases, the magnetic moment increases with the atomic value which
means that small particles are more magnetic than the bulk material.
The magnetic moment of iron (Fe) of nanoparticles is
30% more than that of bulk. At smaller sizes, the clusters become
spontaneously magnetic.
(ii)
The nano-materials shows variation in
their magnetic property when they change from bulk state to cluster
(nano-particle) state.
(iii)
Non-magnetic materials become magnetic
when the cluster size reduces to 80 atoms.
6.2 MECHANICAL
PROPERTIES
(i) In
nanophase materials, the elastic strength is low however, its plastic behavior
is high.
(ii) In
some nanophase materials, it is noted that there is decrease in hardness when
the grain size is less than 10 nm.
However for many nanocrystalline, pure metals (10
nm), the hardness is about 2 to 7 times greater than that of large-grained
(>1 μ m) metals.
(iii)Higher hardness
and mechanical strength (2-7 times) when grain size reduces from 1 μ m
to 10 nm.
(iv)
It has very high ductility and
superplastic behavior at low temperatures.
7 APPLICATIONS OF
NANOPHASE MATERIALS.
1.Materials Technology
We can synthesis harder metals having hardness 5 times higher than normal
metals using nanoparticles.
Stronger, lighter, wear resistant, tougher and flame retardant polymers
are synthesized with nanoparticles as fillers. They are used in replacement of
body parts and metals (bio-materials).
We can produce unusual colour paints using nanoparticles since nanoparticles
exhibit entirely different optical properties.
Nanophase materials are used in annoelectronic devices such as
nanotransistore, ceramic capacitors for energy storage, noise filters and
stabilizers. The special features of these devices include smaller sizes and
reduced power losses.
ZnO thermistors are used in thermal –protection and current-controlling
devices.
2. Information Technology
Nanoparticles are used for data storage.
Quantum electronic devices have started replacing bulk conventional
devices.
Nano materials are used to produce very tiny permanent magnets of high
energy products. Hence, they are used in high-density magnetic recording.
Magnetic devices made of Cu-Fe alloy are used in RAM, READ / WRITE heads
and sensors.
Quantum dots, quantum wells and quantum wires are mainly produced from
semiconductor nanomaterials. Hence, they are used in computer storage (memory)
devices.
3. Biomedicals
Biosensitive nanoparticles are used for tagging of DNA and DNA chips.
Controlled drug delivery is possible using nanotechnology. Diffusion of
medicine through nanoporous polymer reservoir as per the requirement is very
useful in controlling the disease.
Nanostructured ceramics readily interact with bone cells and bence finds
applications as an implant material.
4. Energy storage
Since the hydrogen absorbing capability increases with decrese of size of
nanoparticles, nanoparticles of Ni, Pd and Pt are useful in hydrogen storage
devices.
Metal nanoparticles are very useful in fabrication of ionic batteries.
5. Optical devices
Nanomaterials are used
in making effici
Nanoparticulate zinc oxide is used to manufacture effective Sunscreens.
Nanoparticles are used in the coatings for eye glasses to protect from
scratch or breakage.
6. Transmission
lines
Nanophase materials are used in the fabrication of
signal processing elements such as filters, delay lines, switches etc.
7. Nanomicro-Electro
Mechanical Systems (Nano MEMS) have direct
implications on integrated circuits, optical switches, pressure sensors
and mass sensors.
8. Molecular
Nano-Technology (MNT) is aimed to develop robotic
machines, called assemblers on a molecular scale, molecular-size power
sources and batteries.
9. Underwater
nanosensor networks are used to detect the movement of ships
in an efficient manner with faster response. They can also detect
chemical, biological or radiological materials in cargo containers.
8 NON-LINEAR MATERIALS
AND BIO-MATERIALS
8.1 BIREFRINGENCE AND KERR EFFECT.
The appearance of
double refraction under the influence of an external agent is known as
artificial double refraction or induced birefringence.
Optical Kerr Effect
Anisotropy induced in
an isotropic medium under the influence of an electric field is known as Kerr
effect.
A sealed glass cell
known as Kerr cell filled with a liquid comprising of asymmetric molecules is
used to study the Kerr effect.
Two plane electrodes
are placed in parallel to each other. When a voltage is applied to there electrodes,
a uniform electric field is produced in the cell.
The Kerr cell is placed
between a crossed polarizer system (Fig), When the electric field is applied,
the molecules of the liquid tend to align along the field direction.
As the molecules are asymmetric,
the alignment causes anisotropy and the liquid becomes double refracting. The
induced birefringence is proportional to the square of the applied electric
field E and to the
wavelength λ of
incident light.
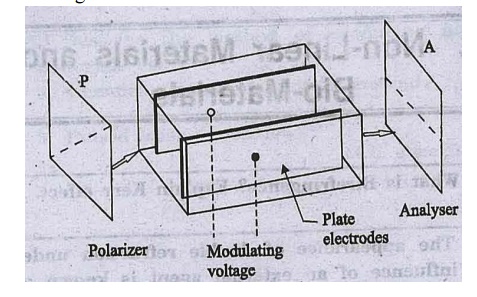
Fig. Kerr effect –Birefringence is
induced in a liquid subjected to an electric field
The change in
refractive influx is given by
∆μ= K λE2
Where
K is known as the Kerr constant
8.2 EXPLAIN NON-LINEAR
PROPERTIES AND SECOND HARMONIC GENERATION. Basic Principle of Non Linear
Properties
We know that a light
wave is electromagnetic in nature ie., it consists of electric and magnetic
fields. When the light propagates through a material, it changes the properties
of the medium, such as the refractive index. It depends on the electric and
magnetic fields associated with the light.
For example, we could
not observe nonlinear effects with the ordinary light beam of low intensity,
since the electric and magnetic fields associated with the light beams is very
weak.
With the invention of
laser, it is now possible to have electric fields which are strong enough to
observe interesting non linear effects.
Thus if electric and
magnetic fields are strong enough, the properties of the medium will be
affected which in turn will affect the propagation of the light beam.
8.3NON
LINEAR PROPERTIES
Few of the nonlinear phenomena observed are
1. Second
harmonic generation
2. Optical
mixing
3. Optical
phase conjugation
4. Soliton
8.4SECOND
HARMONIC GENERATION
In a linear medium,
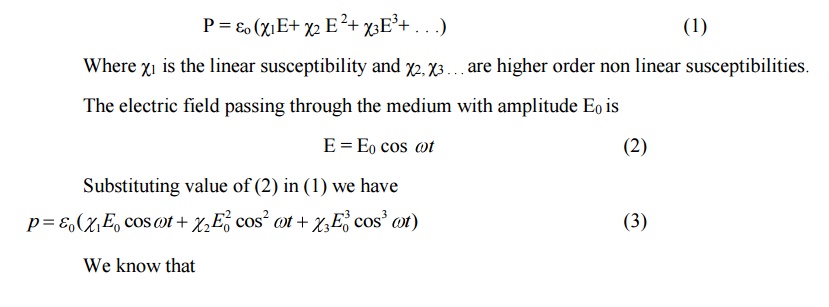
polarization P is directly proportional to the electric field E
P
∝E
P
= εoχE
Whereo ε-
Permittivity of free space
χ -
electrical susceptibility
In nonlinear medium for
higher fields ie., higher intensities of light the non linear effects are
observed.
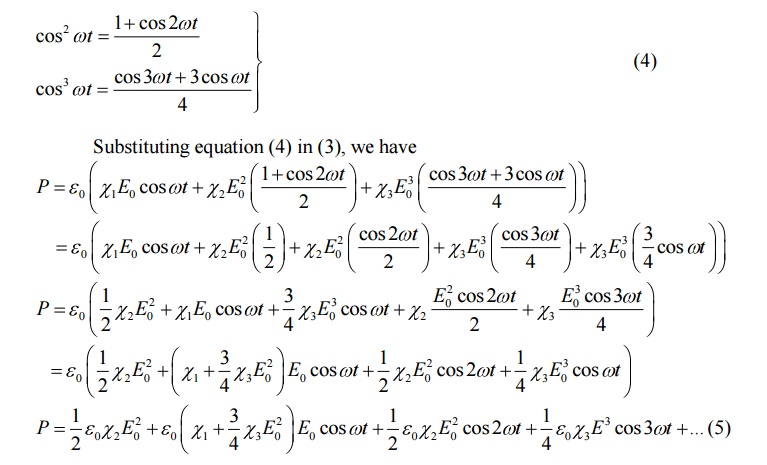
In the above equation,
1st term gives rise to dc field across the medium, the second
term gives external polarization and is called first or fundamental harmonic polarisability.
The third term which
oscillates at a frequency 2w is called second harmonic of polarization and
other terms are referred as higher harmonic polarization.
Both first term (dc
field) and third term (second harmonic of polarization) added together is
called optical rectification.
The second harmonic
generation is possible only the crystals lacking inversion symmetry. SHG
crystals are quartz, potassium dihydrogen phosphate (KDP), Ammonium dihydrogen
phosphate (ADP), Barium titante (BaTiO3) and Lithium lodate (LiIO3)
The observation of
second harmonic generation by KDP is shown in figure.
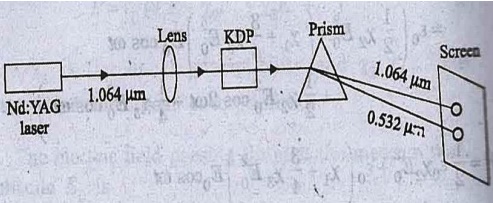
Fig.
Arrangement for observing second harmonic generation
When the fundamental
radiation (1.064 m) from Nd: YAG laser is sent through SHG crystal like KDP,
conversion takes place to double the frequency. i.e., half the wavelength
(0.532 m) takes place.
8.5 BIOMATERIALS WITH THEIR PROPERTIES
AND APPLICATIONS.
The materials which are
used for structural applications in the field of medicine are known as
Biomaterials.
In the recent years,
new biomaterials like nanobiomaterials are emerging up due to the requirements
in the medical field for different applications.
8.6 CLASSIFICATION OF BIOMATERIALS
Based
on the applications in the field of medicine, biomaterials are classified as
1. Metals
and alloys biomaterials
2. Ceramics
biomaterials.
3. Polymer
biomaterials.
4. Composite
biomaterials
Sometimes, a single
material mentioned above cannot fulfill the complete requirements imposed for
specific applications. In such case, combinations of more than one material are
required.
Metals and Alloys
Metals and alloys are
used as biomaterials due to their excellent electrical and thermal conductivity
and mechanical properties.
TYPES OF BIOMATERIALS USING METALS AND
ALLOYS
1. Cobalt
based alloys
2. Titanium
3. Stainless
steel
4. Protosal
from cast alloy
5. Conducting
metals such as Platinum
8.7APPLICATIONS
The metals and alloys biomaterials are used in implant
and orthopedic applications.
1. Stainless
steel is the predominant implant alloy. This is mainly due to its ease
of fabrication and desirable mechanical properties and corrosion resistant.
2. Proposal
from cast alloy of Co –Cr –Mo is used to make stem and used for implant hip
endoprosthesis.
3. The
advanced version of protosal –10 from Co –Ni –Cr –Mo
alloy is widely used in Hip joints, Ankle joints, Knee joints, leg
lengthening spaceas.
4. ASTMF
–136 (composition of Ti –6A1 –4V, EL1 alloy, forged) due to its high strength /
weight ratio, high corrosion resistance and high bio compatibility, this alloy
is used in dental applications for making screws, wires and artificial teeth.
5. Ni
–Ti shape memory alloy is used in dental arch wires, micro surgical
instruments, blood clot filters, guide wires etc.
8.8CERAMICS
Ceramics are used as biomaterials due to their high
mechanical strength and biocompatibility.
Types of Bio-Ceramic materials.
1. Tricalcium
phosphate
2. Metal
oxides such as Al2O3 and SiO2
3. Apatite
ceramics
4. Porous
ceramics
5. Carbons
and Alumina
Applications
1. Ceramic
implants such as Al2O3 and with some SiO2 and
alkali metals are used to make femoral head. This is made from powder
metallurgical process.
2. Tricalcium
phosphate is used in bone repairs.
3. Orthopedic
uses of alumina consists of hip and knee joints, tibical plate, femur shaft,
shoulders, radius, vectebra, leg lengthening spaces and ankle joint prosthesis.
Porous alumina is also used in teeth roots.
4. Apatite
ceramics are new bio active ceramics. They are regarded as synthetic bone,
readily allows bone ingrowth, better than currently used alumina Al2O3.
5. Carbon
has good biocompatibility with bone and other tissues. It has high strengths
and an elastic molecules close to that of bone.
6. Carbon
coatings find wide applications in heart valves, blood vessel grafts,
percutaneous devices because of exceptional compatibility with soft tissues and
blood.
7. Percutaneous
carbon devices containing high density electrical connectors have been used for
the chronic stimulation of the cochlea for artificial hearing and stimulation
of the visual cortex to aid the blind.
Bio Polymers
Biopolymers
are macromolecules (protein, nucleic acids and polysachacides) formed in
nature during the growth cycles of all
organisms.
Biopolymers find
variety of applications as biomaterials. The most prominent among them are
collagens, muco-polysaccharides –chitin, collagens and its derivatives.
Collagnes which are
major animal structural proteins are widely used in a variety of forms such as
solution, gel, fibers, membranes, sponge and tubing for large number of
biomedical applications including drug delivery system, vessels, valves corneal
prosthesis, wound dressing, cartilage substitute and dental applications.
Biomaterials in Opthamology
Biomaterials find
important applications in opthalmology. They are used to improve and maintain
vision. Eye implants are used to restore functionality of cornea, lens, etc,
when they are damaged or diseased.
The biomaterials
include viscoelastic solutions intraocular lenses, contact lenses, eye shields,
artificial tears, vitreous replacements, correction of corneal curvature.
Dental Materials
Polymers, composites,
ceramic materials and metal alloys are four main groups of materials used for
dental applications.
A large number of materials are tested for porous
dental implants, which include stainless steel,Co –Cr –Mo alloy, PMMA,
proplast and Daceon, velour coated metallic implants, porous calcium aluminate
single crystal alumina, bioglass, vitreous and pyrolytic carbons.
The dental applications
include impression materials, dentine base and ceorons, bridges, inlays and
repair or cavities, artificial teeth, repair of alveolar bone, support for
mandible .
Related Topics