Chapter: Modern Pharmacology with Clinical Applications: Estrogens, Progestins, and Specific Estrogen Receptor Modulators (SERMs)
Selective Estrogen Receptor Modulators (SERMs): Clinical Uses
CLINICAL USES
The chief therapeutic uses of
estrogens and progestins are as oral contraceptives and hormone replacement
therapy. Progestins and SERMs are also important agents in the treatment of
osteoporosis, breast cancer, endometrial cancer, and infertility.
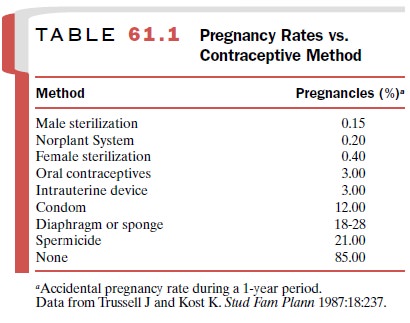
Oral Contraception
Oral contraceptives are among
the most effective forms of birth control (Table 61.1). The most widely used
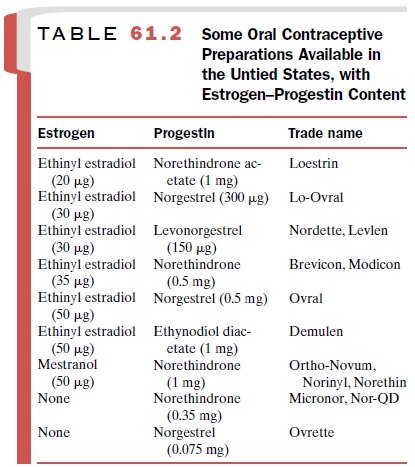
type of oral contraceptive in the United States today is the combination preparation, that is, a
combination of es-trogen and progestin (Table 61.2). Users take a tablet daily
that contains both an estrogen and a progestin for 20 to 21 days of the
menstrual cycle and then nothing or a placebo for the remainder of the cycle or
the next 7 to 8 days. Withdrawal bleeding occurs 2 to 3 days after dis-continuation
of this regimen. Combination preparations vary in the dose of synthetic
estrogen and progestin they contain. The use of sequential and triphasic oral
These preparations are designed to more
closely simulate estrogen-to-progestin ratios that occur physiologically during
the menstrual cycle. Ethinyl estradiol and mestranol are the only two
estrogen con-stituents used for oral contraception in the United States. The
use of ethinyl estradiol is favored. Mestranol is in-active until it is
metabolized to ethinyl estradiol.
Several progestins are used
in combination prod-ucts. Norgestrel (Ovrette)
is a mixture of active and in-active enantiomers; levonorgestrel (Norplant) is the ac-tive enantiomer.
Levonorgestrel and norethindrone are the most potent synthetic progestins in
oral contracep-tive preparations.
Inhibition of ovulation is
the primary mechanism of the contraceptive action of sequential and combination
birth control preparations. Ovulation is prevented by the suppression of the
midcycle surge of FSH and LH. Estrogens are most active in inhibiting FSH
release, but at high enough doses, they also inhibit LH release. In low-dose
combination products, the progestin causes LH suppression. The progestin
component is also important in causing withdrawal bleeding at the end of the
cycle.
Combination oral
contraceptive drugs having the lowest effective concentration of both estrogen
and progestin should be prescribed. These preparations are known as low-dose
oral contraceptive agents. Adverse effects of both estrogen and progestin are
minimized with the use of these agents.
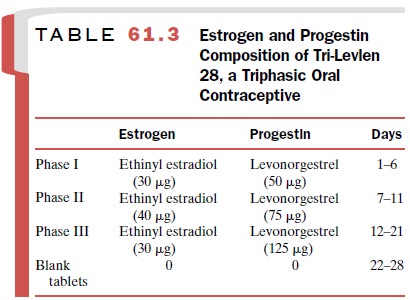
Clinical experience with the
low-dose combination drugs indicates that the estrogen-to-progestin ratio is
critical in achieving maximum contraceptive activity. In certain combinations (Ortho-Novum 7/7/7, Tri-Norinyl, Tri-Levlen, Triphasil), the
estrogen-to-progestin ratio is varied
in three phases over the initial 21 days by chang-ing the progestin content of
the tablets. An example of the estrogen and progestin doses found in this type
of oral contraceptive is shown in Table 61.3.
Progestin-only oral contraceptive formulations consist of a low dose of either norethindrone or norgestrel (Table 61.2).
Because of an
increased incidence of cer-tain side effects and slightly decreased
contraceptive ac-tivity, progestin-only oral contraceptives are not
exten-sively used. The undesirable side effects associated with progestin-only
contraceptives are irregular bleeding episodes, headache, weight gain, and mood
changes. Progestin-only contraceptive devices
are used. The Norplant System for
contraception consists of a series of levonorgestrel-filled
pliable plastic tubes that are im-planted subcutaneously on the inside of the
upper arm by a physician. While one set of six tubes can remain ef-fective for
up to 5 years, the contraceptive effects are readily reversible with removal of
the implant. Adverse effects are similar to those seen with other progestin-only
contraceptives; however, accidental pregnancy is less frequent.
Mirena is a relatively new intrauterine contraceptive device that releases levonorgestrel into the uterine cav-ity for 5
years. Use of this contraceptive device is asso-ciated with fewer systemic
progestin side effects and is at least as effective as Norplant.
Abortifacients and Emergency Contraceptives
Progesterone is a hormone
required for the mainte-nance of pregnancy. Termination of early pregnancy is
effected using the steroidal antiprogestin drug, mifepri-stone (RU486), which
acts by blocking progestin bind-ing to the progesterone receptor. A single oral
dose of RU486 followed by a single dose of a prostaglandin (Misoprostol) 48 hours later is 90 to 95%
effective in terminating pregnancy. The side effects are generally mild except
for heavy bleeding. Severe cardiovascular complications have occurred and may
be due to the prostaglandin component of this treatment. The use of RU486 is
therefore contraindicated in women at risk for cardiovascular disease,
including smokers and women over 35 years of age.
High-dose estrogen and
high-dose progestin are ef-fective in emergency contraception when given
immedi-ately following unprotected coitus. Plan
B is an emer-gency contraceptive kit consisting of two tablets of the
progestin levonorgestrel (0.75 mg). The first tablet must be taken as soon as
possible but no later than 3 days af-ter coitus, and the second tablet is taken
72 hours later. This regimen is more effective and better tolerated than the Preven emergency contraceptive kit, an
estrogen– progestin combination (two tablets of 50 g ethinyl estradiol and two
tablets of 0.25 mg of levonorgestrel). The high doses of estrogen in the Preven regimen are as-sociated with
severe nausea and vomiting.
Hormone Replacement Therapy
The beginning of menopause is
marked by the last men-strual cycle. This is the result of declining ovarian
func-tion and reduced synthesis of estrogens and proges-terone. Estrogen
production in postmenopausal women is usually only about 10% of that in
premenopausal women. Almost no progesterone is synthesized in post-menopausal
women. Hormone replacement therapy (HRT) generally refers to the administration
of estrogen– progestin combinations. Estrogen replacement therapy (ERT)
consists of the use of an estrogen alone, usually in the form of conjugated
equine estrogens or an estro-gen transdermal patch.
The four most common symptoms
associated with menopause are vasomotor disorders, or hot flashes; uro-genital
atrophy; osteoporosis; and psychological distur-bances. A varying proportion of
women may have one or more of these symptoms.
Osteoporosis
One in four postmenopausal
women have osteoporosis. Osteoporosis, a
decrease in bone mass, constitutes the most serious effect of menopause. It
has been estimated that following
cessation of ovarian function, the loss of bone mass proceeds at a rate of 2 to
5% per year. As a result of osteoporosis, as many as 50% of women de-velop
spinal compression fractures by age 75, and 20% will have hip fractures by age
90.
Estrogen replacement therapy
can prevent bone loss and actually increase bone density in postmenopausal
women. Estrogen treatment is the most
effective therapy for osteoporosis and
significantly reduces the incidence of
bone fractures in postmenopausal women. The usual dose of estrogen prescribed
is 0.625 mg/day of conju-gated equine estrogens (Premarin). Alternatively, a transdermal estrogen patch can be used.
Endometrial cancer is not a
concern in women who have undergone hysterectomy. However, in women with an
intact uterus, there is a risk of endometrial cancer with ERT. A preliminary
endometrial biopsy should be performed before instituting therapy to rule out
en-dometrial hyperplasia or cancer, and biopsies should be repeated at 6- to
12-month intervals in women receiving ERT. When endometrial cancer is a
concern, patients should consider HRT. Estrogens should be given in an
intermittent fashion followed by at least 7 to 10 days of treatment with a
progestin alone. Oral norgestimate, norethindrone acetate, and
medroxyprogesterone ac-etate are progestins given to postmenopausal women
re-ceiving estrogens to control endometrial proliferation.
Alternatives to steroid
hormone therapy for osteo-porosis include raloxifene, bisphosphonates, sodium
fluoride, vitamin D and calcium supplementation, calci-tonin, and parathyroid
hormone. Tamoxifen has estro-genic effects on bone and delays bone loss in post-menopausal
women. However as a result of estrogenic activity in the uterus, long-term
tamoxifen adminis-tration has been associated with an increased risk of endometrial
cancer. Raloxifene has estrogenic activity on bone but antiestrogenic activity
in uterus and breast tis-sue. Raloxifene is a SERM that was specifically
approved for the prevention and treatment of osteoporosis.
Cardiovascular Actions
Declining estrogen levels associated with menopause are correlated
with an increased risk of cardiovascular re-lated deaths in women. The protective effects of
estro-gens on the lipid profile are well recognized. There is a relationship
between elevated levels of cholesterol, triglycerides, very low density
lipoproteins, low-density lipoproteins, and coronary artery disease; in
contrast, the elevation of high-density lipoproteins appears to be related to a
reduced incidence of cardiovascular effects. The hormonal effects produced by
estrogen and pro-gestin therapy vary with the dosage, duration, route of administration,
and particular preparation. In general, estrogenic compounds lower levels of
“bad cholesterol” (low-density lipoproteins), while progestins raise
low-density lipoproteins and triglycerides.
The use of HRT for mitigation
of cardiovascular dis-ease is not supported by the most recent clinical
studies. The use of estrogen–progestin combinations in post-menopausal women
was associated with a slight in-crease in coronary artery disease and a
threefold eleva-tion in thromboembolic episodes.
Conjugated equine estrogens (Premarin) are the most commonly used
estrogens in the treatment of menopause-associated vasomotor symptoms and
os-teoporosis. Premarin is a mixture
of estrogen sulfates, including estrone, equilin, and 17- -dihydroequilin. The
sulfate derivatives are orally active and are cleaved within the body to yield
the active, unconju-gated estrogen.
Premphase is an estrogen–progestin combination that introduces a cyclic progestin component. Prem-phase packets consist of a 2-week regimen of daily 0.625-mg conjugated equine estrogens
followed by a 2-week period of a combination of conjugated equine es-trogens
and daily medroxyprogesterone. Esterified es-trogens, primarily sodium estrone
sulfate (Estratab), and estropipate (Ogen) are also used. Several
transder-mal patches deliver estradiol continuously. These prod-ucts differ in
their dose of estradiol: Climara,
0.025 mg/day; Estraderm, 0.05 mg/day;
and Vivelle, 0.0375 mg/day.
Vasomotor Symptoms
Vasomotor disorders (hot
flashes) are common, affect-ing 70 to 80% of postmenopausal women. The cause of
the vasomotor changes appears to be associated with the release of LH after
normal female estrogen levels have fallen. These symptoms occur with variable
frequency but generally disappear without treatment within 2 to 3 years of
onset. Estrogen or progestin therapy is often ef-fective in suppressing
vasomotor symptoms. Short-term estrogen therapy (2 years) for these symptoms is
recom-mended and is not associated with increased cancer risk. Continuous therapy
is usually not required.
Urogenital Atrophy
The tissues of the distal
vagina and urethra are of simi-lar embryonic origin, and both are sensitive to
the trophic action of estrogens. Postmenopausal atrophy of these tissues may
result in painful sexual intercourse, dysuria, and frequent genitourinary
infections. Unlike the vasomotor complaints, these symptoms seldom im-prove if
untreated. Treatment with a combination of minimally effective dosages of an
estrogen and a prog-estin is recommended. Estrogen can be administered orally
or in a topical preparation with equivalent effi-cacy. Progestins are given
orally.
Replacement Therapy in Premenopausal Women
Oophorectomy causes many of
the symptoms seen in menopause. The onset and intensity of vasomotor symp-toms
and osteoporosis, however, may be more severe than in women proceeding into the
more gradual age-associated process of menopause. The regimens for
estrogen–progestin replacement therapy in oophorec-tomized patients are
comparable to those recom-mended for postmenopausal women.
Several genetic conditions
lead to a failure of ovar-ian development. These genetic alterations lead to a
failure in the synthesis of normal amounts of estrogen or progesterone, so that
female secondary sex charac-teristics do not appear at puberty. Only with
estrogen treatment is there stimulation of the growth of the gen-italia, breast
enlargement, and development of female body contours and distribution of body
hair. Some in-creases in body height also occur with estrogen therapy, but this
is more marked after androgen treatment. Replacement estrogens can be
administered using a transdermal patch formulation or micronized estradiol (Estrace, Gynodiol).
Central Nervous System Effects
Insomnia and fatigue in many
postmenopausal women may be related to reduced estrogen levels; there is a
cor-relation between the incidence of waking episodes and low levels of
estrogen. Estrogen replacement therapy may be used to treat severe cases.
There is considerable
interest in the role of estrogen hormone replacement therapy as a cognitive
enhancer in postmenopausal women. Although there is some evi-dence for improved
cognitive abilities in postmeno-pausal women receiving estrogen replacement
therapy, the effects reported thus far are modest.
Infertility
Anovulation, often related to
altered ratios of estrogen to progestin, can be treated with a variety of
agents, in-cluding estrogen–progestin replacement, clomiphene citrate,
bromocriptine, FSH, LH, human chorionic go-nadotropin, and GnRH. Clomiphene
citrate (Clomid, Serophene) and bromocriptine (Parlodel)
are the two most widely used agents.
Induction of Ovulation
Anovulation can be due to an
insufficient release of LH and FSH during the mid phase of the menstrual cycle.
Induction of ovulation by clomiphene citrate is the re-sult of stimulation of
FSH and LH release. The mecha-nism of this action is probably related to the estrogen antagonist properties of clomiphene citrate. Although estrogens generally exert a
negative-feedback inhibi-tion on FSH and LH secretion by means of a
suppres-sion of GnRH from the hypothalamus, clomiphene ex-erts its action by
stimulating secretion of these hormones. Antagonism of this feedback system
results in a surge of FSH and LH secretion, hence ovulation.
Patients with normal or
elevated estrogen levels and normal pituitary and hypothalamic function respond
most frequently to treatment with clomiphene citrate. In this group, the
ovulation rate following clomiphene citrate may be 80%. Clomiphene citrate is
administered on a cyclic schedule. First, menstrual bleeding is in-duced; next
drug is given orally for 5 days at 50 mg/day. Ovulation is expected 5 to 11
days after the dose of clomiphene citrate. Pregnancy rates approach 50 to 80%
after six such treatment cycles, with most pregnan-cies occurring during the
first three treatment cycles. Clomiphene is also used in conjunction with
go-nadotropins to induce ovulation for in vitro fertiliza-tion.
Related Topics