Chapter: Essentials of Anatomy and Physiology: Cell Structures and their Functions
Movement through the cell membrane
Movement through the cell membrane
A. Define diffusion and concentration gradient.
B. Explain the role of osmosis and that of osmotic pressure incontrolling the movement of water across the cell membrane. Compare hypotonic, isotonic, and hypertonic solutions.
C. Define carrier-mediated transport, and compare theprocesses of facilitated diffusion, active transport, and secondary active transport.
D. Describe endocytosis and exocytosis.
Cell membranes are selectively permeable, meaning that they allow some substances, but not others, to pass into or out of the cells. Intracellular material has a different composition than extra-cellular material, and the cell’s survival depends on maintaining the difference. Substances such as enzymes, glycogen, and potas-sium ions (K+) are found at higher concentrations intracellularly, whereas Na+, Ca2+, and Cl− are found in greater concentrations extracellularly. In addition, nutrients must enter cells continually, and waste products must exit. Because of the permeability char-acteristics of cell membranes and their ability to transport certain molecules, cells are able to maintain proper intracellular concen-trations of molecules. Rupture of the membrane, alteration of its permeability characteristics, or inhibition of transport processes can disrupt the normal intracellular concentration of molecules and lead to cell death.
Movement through the cell membrane may be passive or active. Passive membrane transport does not require the cell to expend energy. Active membrane transport does require the cell to expend energy, usually in the form of ATP. Passive membrane
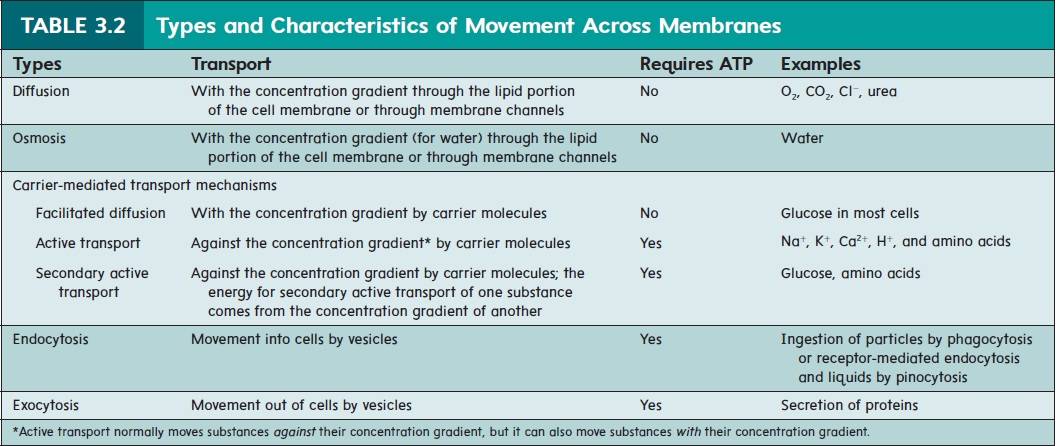
transport mechanisms include diffusion, osmosis, and facilitated diffusion. Active membrane transport mechanisms include active transport, secondary active transport, endocytosis, and exocytosis. Table 3.2 lists the specific types of movement across cell membranes and we discuss each method in detail in the following sections.
Diffusion
A solution is generally composed of one or more substances, called solutes, dissolved in the predominant liquid or gas, which is called the solvent. Solutes, such as ions or molecules, tend to move from an area of higher concentration of a solute to an area of lower concentration of that same solute in solution. This process is called diffusion (figure 3.3, steps 1 and 2). An example of diffu-sion is the distribution of smoke throughout a room in which there are no air currents. Another example of diffusion is the gradual spread of salt throughout a beaker of still water.
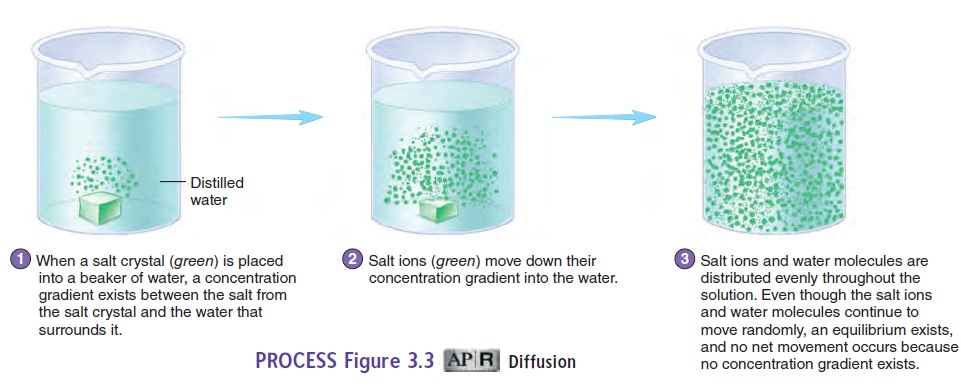
Diffusion results from the natural, constant random motion of all solutes in a solution. More solute particles occur in an area of higher concentration than in an area of lower concentration. Because particles move randomly, the chances are greater that solute particles will move from the higher toward the lower con-centration than from the lower toward the higher concentration. At equilibrium, the net movement of solutes stops, although the random motion continues, and the movement of solutes in any one direction is balanced by an equal movement of solutes in the oppo-site direction (figure 3.3, step 3).
A concentration gradient is the difference in the concentration of a solute in a solvent between two points divided by the distance between the two points. The concentration gradient is said to be steeper when the concentration difference is large and/or the distance is small. When we say that a substance moves down (or with) the concentration gradient, we mean that solutes are diffusing from a higher toward a lower concentration of solutes. When we say that a solute moves up (or against) its concentration gradient, this means that the substance moves from an area of lower solute concentration to an area of higher solute concentration. This sec-ond type of movement does not occur by diffusion and requires energy in order to occur.
In the body, diffusion is an important means of transporting substances through the extracellular and intracellular fluids. In addition, substances, such as nutrients and some waste products, can diffuse into and out of the cell. The normal intracellular con-centrations of many substances depend on diffusion. For example, if the extracellular concentration of O2 is reduced, not enough O2 diffuses into the cell, and the cell cannot function normally.
The phospholipid bilayer acts as a barrier to most water-soluble substances. However, certain small, water-soluble substances can diffuse between the phospholipid molecules of cell membranes. Other water-soluble substances can diffuse across the cell membrane only by passing through cell membrane channels (figure 3.4).
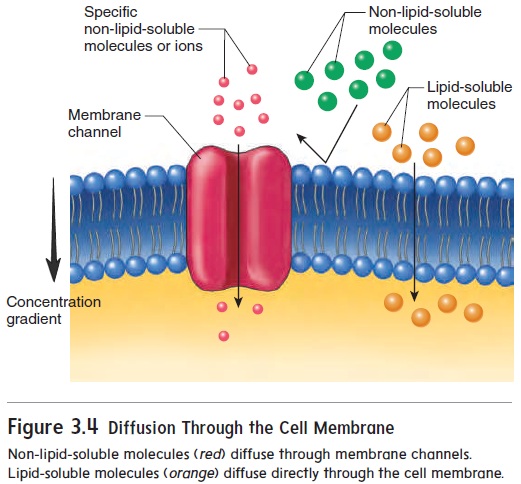
Molecules that are lipid-soluble, such as O2, CO2, and steroids, pass readily through the phospholipid bilayer.
Cell membrane channels consist of large protein molecules that extend from one surface of cell membranes to the other (figure 3.5). There are several channel types, each of which allows only certain substances to pass through. The size, shape, and charge of a molecule all determine whether it can pass througheach kind of channel. For example, Na+ passes through Na+ channels, and K+ and Cl− pass through K+ and Cl− channels, respectively. Rapid movement of water across the cell membrane also occurs through membrane channels.
In addition, cell membrane channels differ in the degree to whichions pass through them. Some channels constantly allow ions to pass through. These channels are called leak channels. Other channelslimit the movement of ions across the membrane by opening and closing. These channels are called gated channels (figure 3.5).

Osmosis
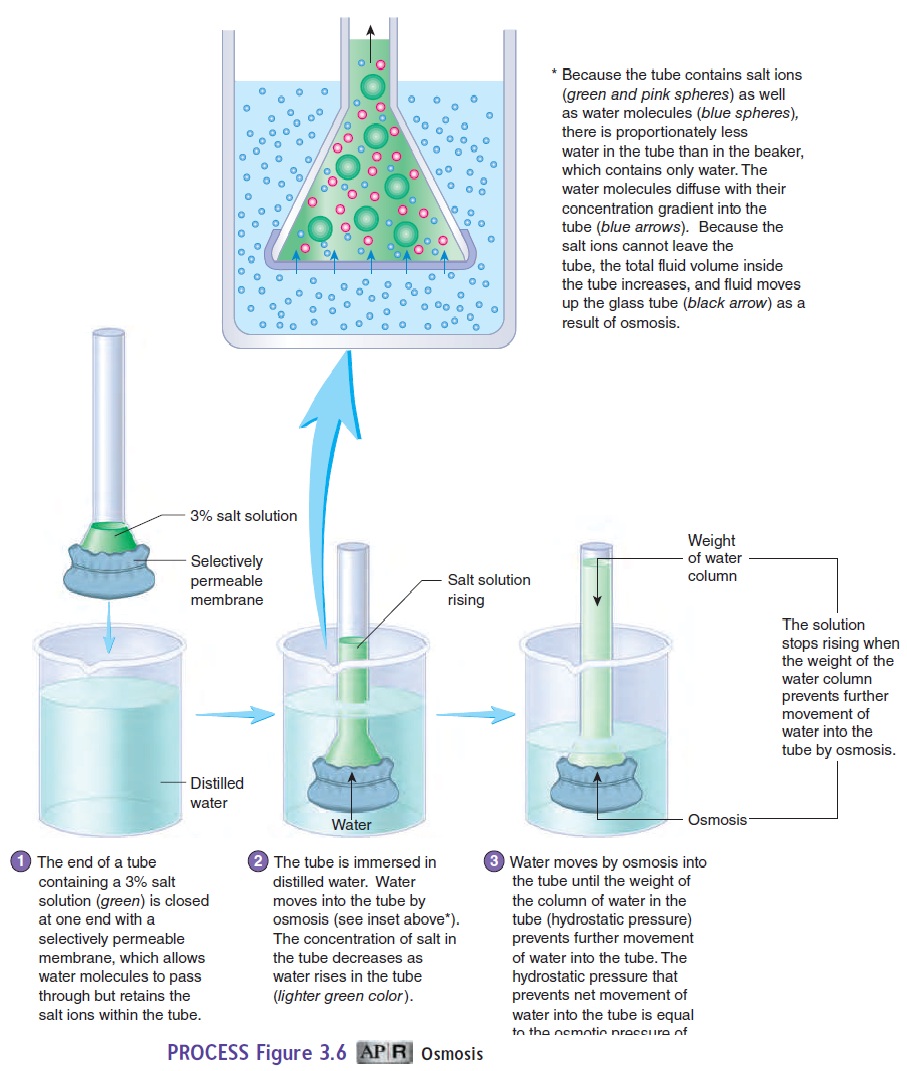
Osmosis (os-mō′\sis) is the diffusion of water (a solvent) acrossa selectively permeable membrane, such as the cell membrane, from a region of higher water concentration to one of lower water concentration (figure 3.6; see table 3.2). Even though water is a polar molecule, it is small enough that it can move across the cell membrane by passing either between the phospholipid molecules or through water channels. Osmosis is important to cells because large volume changes caused by water movement can disrupt nor-mal cell functions. Osmosis occurs when the cell membrane is less \permeable, selectively permeable, or not permeable to solutes and a concentration gradient for water exists across the cell membrane. Water diffuses from a solution with a higher water concentration across the cell membrane into a solution with a lower water con-centration. The ability to predict the direction of water movement across the cell membrane depends on knowing which solution on either side of the membrane has the higher water concentration.
The concentration of a solution, however, is expressed not in terms of water, but in terms of solute concentration. For example, if sugar solution A is more concentrated than sugar solution B, then solution A has more sugar (solute) than solution B. As the concentration of a solution increases, the amount of water (sol-vent) proportionately decreases. Water diffuses from the less concentrated solution B (less sugar, more water), into the more concentrated solution A (more sugar, less water). In other words, water diffuses toward areas of high solute concentration anddilutes those solutes.
Osmotic pressure is the force required to prevent the move- ment of water across a selectively permeable membrane. Thus, osmotic pressure is a measure of the tendency of water to move by osmosis across a selectively permeable membrane. It can be measured by placing a solution into a tube that is closed at one end by a selectively permeable membrane and immersing the tube in distilled water (see figure 3.6, step 1). Water molecules move by osmosis through the membrane into the tube, forcing the solution to move up the tube (see figure 3.6, step 2). As the solution rises, its weight produceshydrostatic pressure (see figure 3.6, step 3), which moves water out of the tube back into the distilled water surrounding the tube. Net movement of water into the tube stops when the hydrostatic pressure in the tube causes water to move out of the tube at the same rate at which it diffuses into the tube by osmosis. The osmotic pressure of the solution in the tube is equal to the hydrostatic pressure that prevents net movement of water into the tube.
The greater the concentration of a solution, the greater its osmotic pressure, and the greater the tendency for water to move into the solution. This occurs because water moves from less concentrated solutions (less solute, more water) into more concentrated solutions (more solute, less water). The greater the concentration of a solution, the greater the tendency for water to move into the solution, and the greater the osmotic pressure must be to prevent that movement.
When placed into a solution, a cell may swell, remain unchanged, or shrink, depending on the concentration gradient between the solution and the cell’s cytoplasm. A hypotonic (hı̄′pō-ton′ik; hypo,under) solution usually has a lower concentration of solutes and a higher concentration of water relative to the cytoplasm of the cell. Thus, the solution has less tone, or osmotic pressure, than the cell. Water moves by osmosis into the cell, causing it to swell. If the cell swells enough, it can rupture, a process called lysis (li′sis) (figure 3.7a). When a cell is immersed in an isotonic (ı̄′sō-ton′ik; iso, equal) solution, the concentrations of various sol-utes and water are the same on both sides of the cell membrane. The cell therefore neither shrinks nor swells (figure 3.7b). When a cell is immersed in a hypertonic (hi′per-ton′ik; hyper, above) solution, the solution usually has a higher concentration of solutes and a lower concentration of water relative to the cytoplasm of the cell. Water moves by osmosis from the cell into the hypertonic

Osmosis
solution, resulting in cell shrinkage, or crenation (krē-nā′shŭn) (figure 3.7c). In general, solutions injected into blood vessels or into tissues must be isotonic to the body’s cells because swelling or shrinking disrupts normal cell f\unction and can lead to cell death.
Carrier-Mediated Transport Mechanisms
Many nutrient molecules, such as amino acids and glucose, cannot enter the cell by diffusion. Likewise, many substances produced in cells, such as proteins, cannot leave the cell by diffusion.
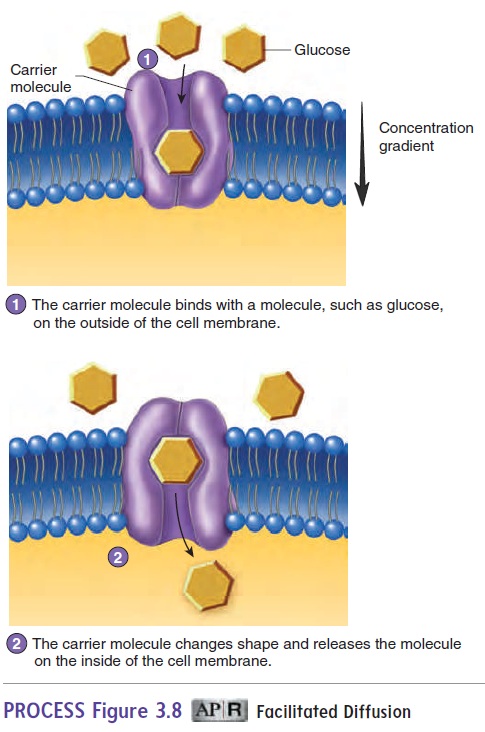
Carrier molecules, which are proteins within the cell membrane,are involved in carrier-mediated transport mechanisms, which move large, water-soluble molecules or electrically charged ions across the cell membrane. A molecule to be transported binds to a specific carrier molecule on one side of the membrane. The bind-ing of the molecule to the carrier molecule in the cell membrane causes the three-dimensional shape of the carrier molecule to change, and the transported molecule is moved to the opposite side of the cell membrane. The transported molecule is then released by the carrier molecule, which resumes its original shape and is avail-able to transport another molecule. Carrier-mediated transport mechanisms exhibit specificity; that is, only specific molecules are transported by the carriers. There are three kinds of carrier-mediated transport: facilitated diffusion, active transport, and secondary active transport.
Facilitated Diffusion
Facilitated diffusion is a carrier-mediated transport processthat moves substances across the cell membrane from an area of higher concentration to an area of lower concentration of that substance (figure 3.8; see table 3.2). Because movement is with the concentration gradient, metabolic energy in the form of ATP is not required.
Active Transport
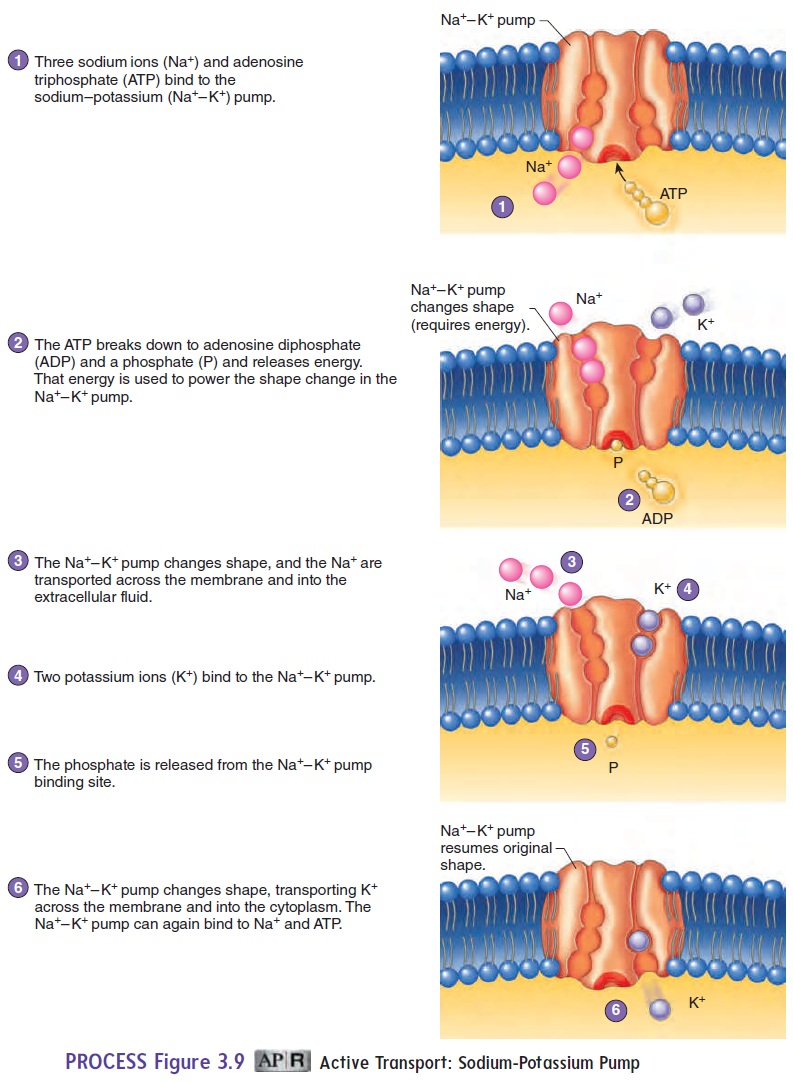
Active transport is a carrier-mediated process that moves substancesacross the cell membrane from regions of lower concentration to those of higher concentration against a concentration gradient (see table 3.2). Consequently, active transport processes accumulate substances on one side of the cell membrane at concentrations many times greater than those on the other side. These dramatic concentration differences are important for normal cell activity. Active transport requires energy in the form of ATP; if ATP is not available, active transport stops. One example of active transport is the movement of various amino acids from the small intestine into the blood. The malfunction of active transport can lead to serious health conditions. Cystic fibrosis is a genetic disorder that affects the active transport of Cl− into cells, as described in the Clinical Impact essay later.
In some cases, the active transport mechanism can exchange one substance for another. For example, the sodium-potassiumpump moves Na+out of cells and K+into cells (figure 3.9). Theresult is a higher concentration of Na+ outside the cell and a higher concentration of K+ inside the cell. The concentration gradients for Na+ and K+, established by the sodium-potassium pump, are essential in maintaining the resting membrane potential .
Secondary Active Transport
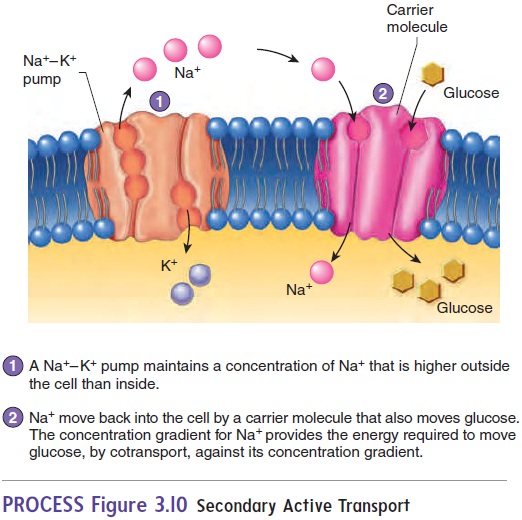
Secondary active transport involves the active transport of onesubstance, such as Na+, across the cell membrane, establishing a concentration gradient. The diffusion of that transported substance down its concentration gradient provides the energy to transport a second substance, such as glucose, across the cell membrane (figure 3.10). In cotransport, the diffusing substance moves in the same direction as the transported substance; in countertransport, the diffusing substance moves in a direction opposite to that of the transported substance.
Endocytosis and Exocytosis
Large water-soluble molecules, small pieces of matter, and even whole cells can be transported across cell membranes in membrane-bound sacs called vesicles. Because of the fluid nature of membranes, vesicles and cell membranes can fuse, allowing the contents of the vesicles to cross the cell membrane.
Endocytosis (en′dō-sı̄-tō′sis) is the uptake of material through the cell membrane by the formation of a vesicle (see table 3.2). The cell membrane invaginates (folds inward) to form a vesicle containing the material to be taken into the cell. The vesicle then moves into the cytoplasm.
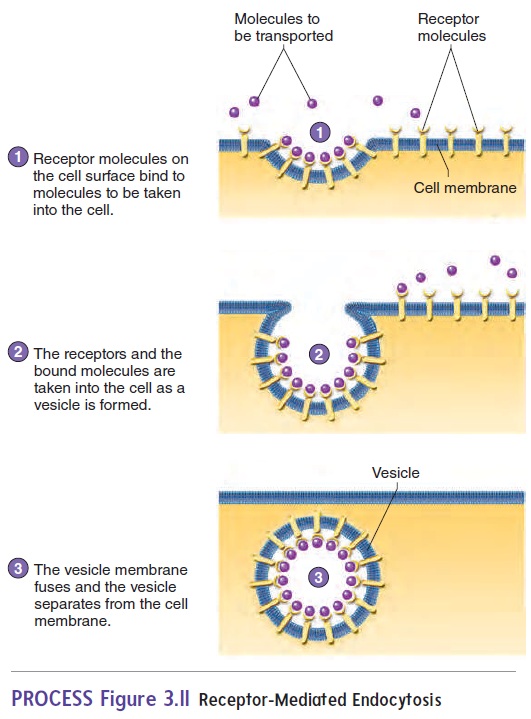
Endocytosis usually exhibits specificity. The cell membrane contains specific receptor molecules that bind to specific sub-stances. When a specific substance binds to the receptor molecule,endocytosis is triggered, and the substance is transported into the cell. This process is called receptor-mediated endocytosis(figure 3.11). Cholesterol and growth factors are examples of molecules that can be taken into a cell by receptor-mediated endocytosis. Bacterial phagocytosis is also receptor-mediated.
The term phagocytosis (fag′ō-sı̄-tō′sis; cell-eating) is often used for endocytosis when solid particles are ingested. A part of the cell membrane extends around a particle and fuses so that the particle is surrounded by the membrane. That part of the membrane then “pinches off” to form a vesicle containing the particle. The vesicle is now within the cytoplasm of the cell, and the cell membrane is left intact. White blood cells and some other cell types phagocytize bacteria, cell debris, and foreign particles. Phagocytosis is an important means by which white blood cells take up and destroy harmful substances that have entered the body. Pinocytosis (pin′ō-sı̄-tō′sis; cell-drinking) is distinguished fromphagocytosis in that much smaller vesicles are formed, and they contain liquid rather than particles.
In some cells, membrane-bound sacs called secretory vesicles accumulate materials for release from the cell. The secretory vesicles move to the cell membrane, where the membrane of the vesicle fuses with the cell membrane, and the material in the vesicle is eliminated from the cell. This process is called exocytosis (ek′sō-sı̄-tō′sis; exo, outside) (figure 3.12; see table 3.2). Examples of exocytosis are the secretion of digestive enzymes by the pancreas and the secretion of mucus by the salivary glands.
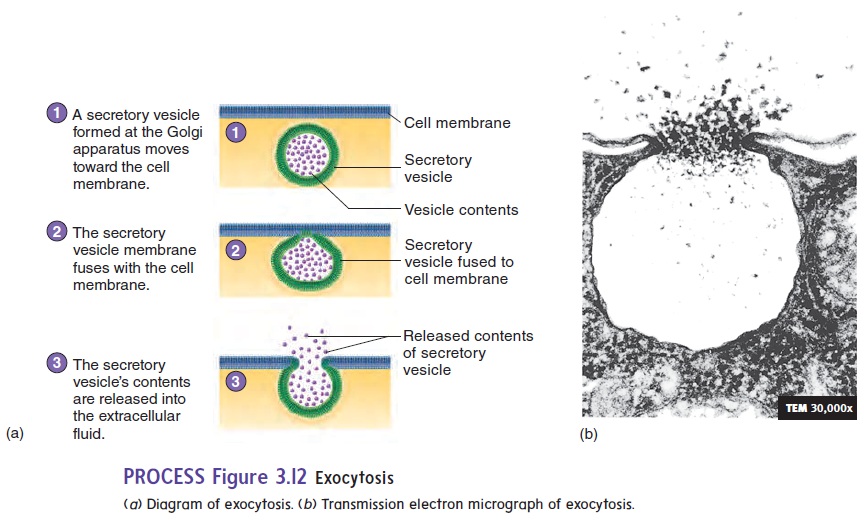
To sum up, endocytosis results in the uptake of materials by cells, and exocytosis allows the release of materials from cells. Vesicle formation for both endocytosis and exocytosis requires energy in the form of ATP.
Related Topics