Chapter: Physics : Advanced Engineering Materials Metallic Glasses
Modern Engineering Materials
Modern Engineering Materials
1 Introduction
2 Metallic Glasses
2.1 Glass transition temperature
2.2 Methods of production of Metallic Glasses
2.3 Types of Metallic Glasses
2.4 Properties of Metallic glasses
2.5 Applications of Metallic glasses
3 Shape Memory Alloys
3.1 Definition
3.2 Working Principle of SMA
3.3 Characteristics of SMA
3.4 Properties of Ni – Ti alloy
3.5 Advantages of SMA’s
3.6 Disadvantages of SMA’s
3.7 Applications of SMA’s
4 Nano Materials
4.1 Introduction
4.2 Definitions
4.3 Synthesis of Nanomaterials
4.4 Chemical Vapour Deposition (CVD)
5 Properties of Nanoparticles
6 Applications of Nanoparticles
7 Non linear materials (NLO materials)
7.1 Higher Harmonic Generation
7.2 Experimental Proof
7.3 Optical mixing
8 Biomaterials
8.1 Biomaterials Classifications
8.2 Conventional implant devices
8.3 Biomaterials Properties
8.4 Modern Engineering MaterialsBiomaterials
Applications
1
INTRODUCTION
There have been a number of science fields which have helps to
producing new engineering materials. Some of the fields are the nano
engineering and the forensic engineering. Hundreds and hundreds of scientists
and inventors are working and experimenting continuously to make this world a
better place to live.
These new inventions have gradually changed the course of living of
people, these New engineering materials are not a result of single engineering
technology but these are obtained or produced from a blend of different
technologies.
Some of the Modern Engineering Materials like Metallic glasses,
Shape memory alloys, Nano materials are discussed here.
2
METALLIC GLASSES
Metallic glasses are the amorphous metallic solids which have high
strength, good metallic properties and between corrosion resistance and will
possess both the properties of metals and glasses.
Example: Alloys of Fe, Ni, Al, Mn, Cu.
2.1
Glass transition temperature
It is an important parameter for the
preparation of metallic glasses. It is defined as a temperature at which the
liquid like atomic structure is obtained into a solid.
The
value of glass transition temperature for metallic alloys is about 20OC
to 30OC.
2.2
Methods of production of Metallic Glasses
Metallic glasses are manufactured by the
following methods. They are,
Twin
roller technique
Melt
extraction technique
Melt
spinning technique
Twin roller system
In this technique, the molten alloy is passed through two rollers
rotating in opposite directions.
2. Melt extraction technique
In this technique, the fast moving roller sweeps off molten droplet
into a strip from a solid rod.
3. Melt spinning technique
Principle
Quenching is a technique used to from metallic glasses. Quenching
means Rapid Cooling. Due to rapid cooling, atoms are arranged irregularly and
from metallic glasses.
Construction
It
consists of a refractory tube with fine nozzle at the bottom. The refractory
tube is placed over the rotating roller. The roller is rotated at a higher
speed to generate a velocity of more than 50 ms 1 . An induction
heater is wounded over the refractory tube to heat the alloy to very high
temperature.
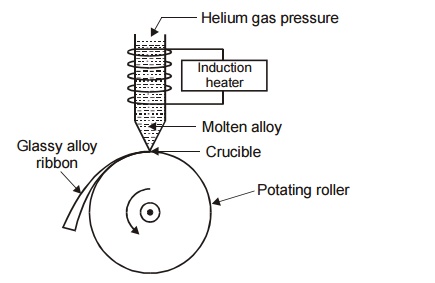
Fig. 5.1 Melt spinning technique
Working
The alloy is put into the refractory tube and induction heater is
switched on. This heats the alloy to very high temperature, hence the super
heated molten alloy ejected through the nozzle on the rotating roller and is
suddenly made to cool. The ejection rate may be increased by increasing the
inert helium gas pressure inside the refractory tube. Thus due to rapid
quenching a glassy alloy ribbon called metallic glass is formed over the
rotating roller.
2.3
Types of Metallic Glasses
The metallic glasses are classified into two
types.
Metal –
Metalloid Glasses
Example: Fe, Co, Ni – Ge, Si, B, C.
Metal –
Metal Glasses Example: Ni, Mg, Cu – Zn, Zr.
Metal – Metalloid Glasses
The first class of metallic glasses is from transition metals (Fe,
Co, Ni) Metalloid (B, Si, C & P) so they are called metal – metalloid glasses.
2. Metal – Metalloid Glasses
Nickel –
Niobium (Ni – Nb)
Magnesium
– Zinc (Mg – Zn)
Copper –
Zirconium (Cu – Zr)
Hafnium
– Vanadium (Hf – V) alloys
2.4 Properties of Metallic glasses
Metallic
glasses have very high strength and are stronger than metals because the
absence of grain boundaries and dislocations.
The
structure of metallic glass is Tetrahedral Close Packing (TCP).
These
are having very high corrosion resistance.
They
have high workability and ductility.
The
electrical resistivity is found to be high (greater than 100)/ due to this eddy
current loss is very small.
Metallic
glasses have both soft and hard magnetic properties.
These
are highly reactive and stable.
It can
also act as a catalyst.
Structural
Properties
They do
not have any crystal defects such as grain boundaries and dislocations.
They
have tetrahedral packed structure. These materials do not passes long range
anisotropy
Mechanical
Properties
Metallic
glasses are stronger than metals and alloyes because they are free from defects
and dislocations.
They
have high corrosion resistance due to random ordering.
They
have high elasticity and ductility.
Electrical
Properties
Electrical
resistivity of metallic glasses is high and it does not vary with temperature.
Eddy
current loss is very small due to high resistivity.
The Hall
co-efficient of metallic glasses is found to have both positive and negtive
signs.
Magnetic
Properties
It obeys
both soft and hard magnetic properties.
The core
losses of metallic glasses are very small.
Chemical
Properties
They
have high corrosion reistance.
They
have catalytic properties.
They are
highly reactive and stable.
2.5 Applications of Metallic glasses
Metallic
glasses are used as reinforcing elements in concrete, plastic and rubber.
Metallic
glasses are used to make pressure vessels and to construct larger fly wheels
for energy storage.
They are
used to make accurate standard resistors, Magnetic resistance sensors and
computer memories.
These
are used in tape recorder heads, cores of high power transformers and magnetic
shields.
Metallic
glasses are used as core in motors.
These
are used to make razor blades and different kinds of springs.
Metallic
glasses can be used as superconductor for producing high magnetic fields and
magnetic levitation effect.
Metallic
glasses are used to make containers for nuclear waste disposal and magnets for
fusion reactors.
Metallic
glasses are used in marine cables, chemical filters, inner surfaces of reactor
vessels, etc.,
10.Metallic glasses are very useful to make surgical
instruments.
11.Superconducting mettalic
glasses are used to produce high magnetic fields and magnetic levitation
effect.
Appendix
The
reasons for choosing metallic glasses are transformer core
Metallic glasses are available in thin sheets therefore the size
and weight of the transformer is reduced. Hysteresis loss is directly
proportional to the area of the hysteresis loop. The loop area of the metallic
glasses is very small and also has high initial permeability. So, the
hysteresis loss is almost zero… The eddy current in the core is inversely
proportional to the resistivity of the core material and directly proportional
to the thickness of the lamination of the core.
Since the resistivity of the metallic glasses is high and the
thickness of the core laminated core material due to small thickness, smaller
area, less weight, high resistivity, soft magnet with low hysteresis and eddy
current losses.
3 SHAPE
MEMORY ALLOYS
Share memory alloys (SMA’s) are metals, which exhibit two very
unique properties, pseudo-elasticity and the shape memory effect. Arne Olander
first observed these unusual properties in 1938 (Oksuta and Wayman 1998), but
not until the 1960’s were any serious research advances made in the field of
shape memory alloys. The most effective and widely used alloys include NiTi
(Nickel – Titanium), CuZnA1 and CuA1Ni.
3.1
Definition
The
ability of the metallic alloys to retain to their original shape when heating or
cooling is called as Shape Memory Alloys (SMA).
These metallic alloys exhibit plastic nature when they are cooled
to very low temperature and they return to their original nature when they are
heated. This effect is known as Shape Memory Effect.
It is also called as smart materials or intelligent materials or
Active materials. There are two types of shape memory alloys,
One way
shape memory – It returns to its memory only when heating
Two way
shape memory – It returns to its memory on both heating and Cooling.
Classification
Piezo
electric SMA materials.
Electrostrictive
SMA materials.
Magnetostrictive
SMA materials.
Thermo
elastic SMA materials.
Examples
: Ni-Ti (Nickel – Titanium), Cu
Zn A1, Cu A1 Ni, Au – Cd, Ni-Mn-Ga and Fe based alloys.
3.2 Working Principle of SMA
The shape memory effect occurs in alloys due to change in the
crystalline structure of the materials with the change in temperature and
stress.
The shape memory effect occurs between two temperature states known
as Martensite and Austenite. The Martensite structure is a low temperature
phase and is relatively soft, It has platelet structure the Austenite is a high
temperature phase and is hard it has needle like structure.
Martensite is the relatively soft and easily deformed phase of
shape memory alloys which exists at lower temperatures. It has two molecular
structures namely, twinned Martensite and deformed Martensite. Austenite is the
stronger phase of shape memory alloys which occurs at higher temperatures, the
shape of the Austenite structure is cubic.
When we apply a constant load on a shape memory alloy and cool it,
its shape changes due to produced strain. During the deformation, the
resistivity, thermal conductivity, Young’s modulus and yield strength are
decreased by more than 40%.
Twinned Martensite state alloy becomes deformed Martensite when it
is loaded. The deformed Martensite becomes Austenite when it is heated, the
Austenite transformed to original twinned Martensite state when it is cooled.
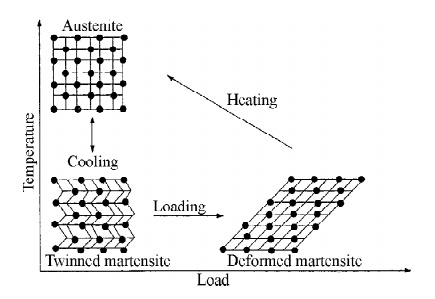
Fig.5.2 Material crystalline arrangement during
shape memory effect
3.3
Characteristics of SMA
1. Hysteresis
Hysteresis of a SMA is defined as the difference between the
temperatures at which the material is 50% transformed to austenite when heating
and 50% transformed to martensite when cooling.
When the temperature is decreased in a metallic material, the phase
transformation takes place from austenite to martensite. This transformation
takes place not only at a single temperature, but over a range of temperatures.
The hysteresis curve for a shape memory alloy is shown below.
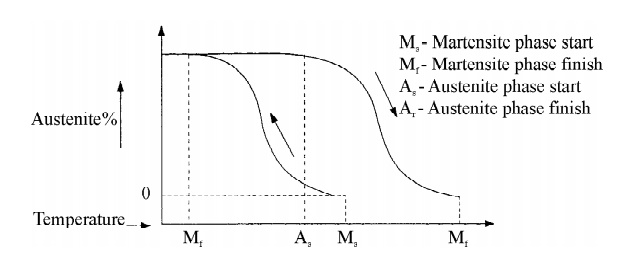
Fig.5.3 Hysteresis curve for
SMA’s
2. Pseudo elasticity
When a metallic material is cooled from a temperature T to a lower
temperature TC it deforms and changes its shape. On reheating the
material to Temperature (T) the shape change is received so that the material
returns to its original state. This effect is known as pseudo elasticity or
thermo elastic property.
3. Super elasticity
Super elasticity is a property of SMA. When a material is deformed
at a temperature slightly greater than its transformation temperature super
elasticity property appears (Rubber like property).
3.4
Properties of Ni – Ti alloy
Ni – Ti is a compound of Nickel and Titanium and it finds many
applications in the field of engineering due to the following properties.
It has
greater shape memory strain.
It has
more thermal stability and excellent corrosion resistance.
It has
higher ductility and more stable transformation temperatures.
It has
better bio-compatibility and it can be electrically heated.
3.5 Advantages of SMA’s
They
ahve good bio-Compatibility.
They
have simplicity, Compactness and high safety mechanism.
They
have good mechanical properties and strong corrosion-resistance.
They
have high power and weigh ratio.
3.6 Disadvantages of SMA’s
They
have poor fatigue properties.
They are
expensive and difficult to preparing in a machine.
They
have low energy efficiency.
They
have limited band with due to heating (or) cooling.
3.7 Applications of SMA’s
Eye glass frames : We know that the recently manufactured eye
glass frames can be bent back and
forth and can retain its original shape within fraction of time.
Toys : We might have seen toys such as butterflies, snakes etc., which are movable and flexible.
Helicopter blades: The life time of helicopter blades depends on
vibrations and their return to its
original shape. Hence shape memory alloys are used in helicopter blades.
Coffee Valves : Used to release the hot milk and the
ingredients at a certain temperature
Medical Applications of SMA’s
It is
used as Micro – Surgical instruments.
It is
used as dental arch wires.
It is
used as flow control devices.
It is
used as ortho – dentil implants.
It is
used for repairing of bones.
They are
used to correct the irregularities in teeth.
Engineering Applications of SMA’s
It is
used as a thermostat valve in cooling system.
It is
used as a sealing plug for high pressure.
It is
used as a fire safety valve.
It is
used for cryofit hydraulic pipe couplings.
It is
used for eye glass frame, toys, liquid safety valve.
It is
used to make microsurgical instruments, orthopedic implants.
It is
used as blood clot filter and for fracture pulling.
It is
used to make antenna wires in cell phones.
It can
be used as circuit edge connector.
4 NANO MATERIALS
4.1
Introduction
Nanomaterials (nanocrystalline materials) are materials possessing
grain sizes of the order of a billionth of a meter. They manifest extremely
fascinating and useful properties, which can be exploited for a variety of
structural and non structural applications.
All materials are composed of grains, which in turn comprise many
atoms. These grains are usually invisible to the naked eye, depending on their
size. Conventional materials have grains varying in size anywhere from 100’s of
microns ( m ) to millimeters (mm). A micron ( m ) is a micrometer or a
millionth (10–6) of a meter. An average human hair is about 100 m in
diameter. A nanometer (nm) is even smaller a dimension than a m and is a
billionth (10–9) of a meter. A nanocrystalline material has grains
on the order of 1-100 nm. The average size of an atom is on the order of 1 to angstroms
( Ao ) in radius.
nanometer comprises 10 Ao , and
hence in one nm, there may be 3-5 atoms, depending on the atomic radii.
Nanocrystalline materials are exceptionally strong, hard, and ductile at high
temperatures, wear-resistant, corrosion-resistant, and chemically very active.
Nanocrystalline materials, or Nanomaterials, are also much more formable than
their conventional, commercially available counterparts.
4.2
Definitions
Nanotechnology
Nanotechnology
is a field of applied science and technology which deals with the matter on the
atomic and molecular scale, normally 1 to 100 nanometers, and the fabrication
of devices with critical dimensions that lie within that size range.
Nanomaterials
Nano materials are the materials with grain sizes of the order of
nano meter (10 9 m) i.e., (1-100 nm). It may be a metal,
alloy, inter metallic (or) ceramic.
Nanomaterials are the materials with atoms
arranged in nano sized clusters which become the building block of the
material. Any Material with a size between 1 and 100 nm [ 10 9 m to 10 7 m] is also called
Nanomaterials.
4.3
Synthesis of Nanomaterials
The Nano mateirals can be synthesized by two
processes, they are
Top –
down approach
Bottom –
up approach
1. Top – down approach
The removal or division of bulk material or the miniaturization of
bulk fabrication processes to produce the desired nanostructure is known as
top-down approach. It is the process of breaking down bulk material to Nano
size.
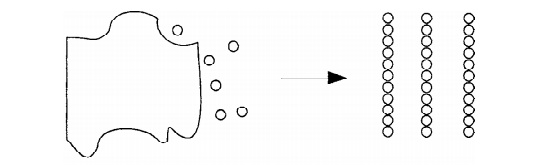
Fig.5.4 Synthesis of
Nanomaterials for Top – down approach
Types of
Top – down Methods
Milling
Lithographic
Machining
Bottom – up approach
Molecules and even nano particles can be used as the building block
for producing complex nanostructures. This is known as Bottom – up approach.
The Nano particles are made by building atom by atom.
Fig.5.5 Synthesis of
Nanomaterials for Bottom – up approach
Types of
Bottom up Methods
Vapour
phase deposition Method
Molecular
beam epitaxy Method
Plasma
assisted deposition Method
Metal
Organic Vapour Phase Epitaxy [MOVPE]
Liquid
phase process [Colloidal method and Sol – Gel method]
4.4
Chemical Vapour Deposition (CVD)
It is method in which the reaction or thermal decomposition of gas
phase species at higher temperatures [500 – 1000OC] and then
deposition on a substrate takes place, in CVD method carbon nanotubes are grown
from the decomposition of hydrocarbons at temperature range of 500 to 1200OC.
In this process, the substrate is placed inside a reactor to which a number of
gases are supplied. The fundamental principle of the process is that a chemical
reaction takes place between the source gases. The produce of that reaction is
a solid material with condenses on all surfaces inside the reactor.
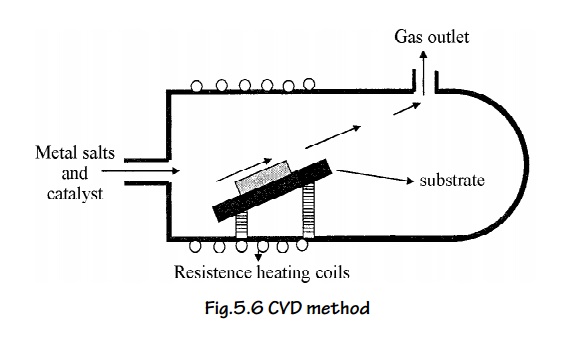
Fig.5.6 CVD method
Example of CVD technique
An aerosol spray pyrolysis (Pyrolysis is the chemical decomposition
of organic materials by heating in the absence of oxygen or any other reagents)
is the example of CVD technique, in which high aqueous metal salts are sprayed
in the form of fine mist and then passed into a hot flow tube. In the hot flow
tube pyrolysis converts the salts into the final products on the substrate, in
this method the materials are mixed in a solution, homogeneous mixing is
obtained to the atomic level. They pyrolysis at low temperature gives the
particles in the size range 5 – 500 nm, in this CVD method catalysts are used
for better chemical reactions, when the catalyst is in nanosize, dispersion of
particles is happened due to templating effect. In the Production of carbon
nanotubes using the decomposition of ethane with hydrogen, Fe, Co or Ni based
catalysts are used. The size and distribution of the catalyst particles
determine the internal diameter of the nanotubes.
5
PROPERTIES OF NANOPARTICLES
A Bulk materials is reduced to a nano size, the
following changes are occurred
Large
fraction of surface atoms
High
surface energy
Spatial
confinement
Reduced
imperfections
The surface area effects, quantum effects can begin to dominate the
properties of matter as size is reduced to the nano scale. These can affect the
optical, electrical and magnetic behavior of materials.
Due to their small dimensions, nano materials have extremely large
surface area to volume ratio, which makes a large fraction of atoms of the
materials to be the surface or interfacial atoms, resulting in more “surface”
dependent material properties.
When the size of their structural components decreases, there is
much greater interface area within the material this can greatly affect both
mechanical and electrical properties, ie., the interface area within the
material greatly increases, which increases its strength. Reduced imperfections
are also an important factor in determination of the properties of the nano
materials
1. Electrical Properties of Nanomaterials
Nanomaterials can store more electrical energy than the bulk
material, because of their large grain boundary (surface) area.
The energy band structure and charge carried density in the nano
materials can be modified quite differently form their bulk size in turn will
modify the electronic properties of the materials.
In nano size an optical absorption band can be introduced, or an
existing band can be altered by the passage of current or by the application of
an electric field.
2. Optical Properties of Nanomaterials
The quantum confinement of electrical carriers within nanoparticles
makes efficient energy and charge transfer over nanoscale distances in nano
devices.
The linear and nonlinear optical properties of nanomaterials can be
finely modified by controlling the crystal dimensions
The color of nanomaterials is changed when the surface plasmon size
is reduced. A surface plasmon is a natural oscillation of the electron gas
inside a given nano sphere.
3. Chemical Properties of Nanomaterials
The increased surface area of nano particle increases the chemical
activity of the material. Metallic nanoparticles can be used as very active
catalysts. Chemical sensors from nanoparticles and nano wires enhanced the
sensitivity and sensor selectivity
4. Mechanical Properties of Nanomaterials
The mechanical properties such as hardness and elastic modulus,
fracture toughness, scratch resistance, fatigue strength, tensile strength,
strain-to-failure, Young’s modulus, impact strength and increased at the nanometer
scale
Energy dissipation, mechanical coupling and mechanical
nonlinearities are also influenced at the nanometer scale.
The strength of the material at nanosize approaching the
theoretical limit due to the absence of internal Structural imperfections such
as dislocations, micro twins, and impurities.
5. Magnetic Properties of Nanomaterials
The strength of a magnet is measured in terms of coercivity and
saturation magnetization values. These values increase with a decrease in the
grain size and an increase in the specific surface area (surface area per unit
volume) of the grains.
6. Thermal Properties of Nanomaterials
Photon transport within the materials will be changed significantly
due the photon confinement and quantization of photon transport, resulting in
modified thermal properties. For example, nano wires from silicon have a much
smaller thermal conductivities compared to bulk silicon.
The nanomaterials structures with high interfaces densities would
reduce the thermal conductivity of the materials. There are several effects
being considered for the reduction of thermal conductivities: interfacial
roughness, phonon band gaps, dispersion mismatch, doping, structural defects,
processing conditions, etc.
6
APPLICATIONS OF NANOPARTICLES
Though
nano – particles are very small, they are the important materials to built
future world. They have applications almost in all engineering fields as
follows Mechanical Engineering
Since
they are stronger, lighter etc., they are used to make hard metals.
Smart
magnetic fluids are used in vacuum seals, magnetic separators etc.
They are
also used in Giant Magneto Resistant (GMR) spin valves.
Nano-
MEMS (Micro-Electro Mechanical Systems) are used in ICs, optical switches,
pressure sensors, mass sensors etc.
Electrical, Electronics and Communication
Engineering
Orderly
assembled nanomaterials are used as quantum electronic devices and photonic
crystals.
Some of
the nanomaterials are used as sensing elements. Especially the molecular
nanomaterials are used to design the robots, assemblers etc.
They are
used in energy storage devices such as hydrogen storage devices, magnetic
refrigeration and in ionic batteries.
Dispersed
nanomaterials are used in magnetic recording devices, rocket propellant, solar
cells, fuel cells, etc.
Recently
nano-robots were designed, which are used to remove the damaged cancer cells
and also to modify the neutron network in human body.
Computer Science Engineering and IT
Nano-materials
are used to make CD’s and semiconductor laser.
These
materials are used to store the information in smaller chips.
They are
used in mobiles, lap – tops etc
Further
they are used in chemical / Optical computers.
Nano –
dimensional photonic crystals and quantum electronic devices plays a vital role
in the recently developed computers.
Bio–Medical and Chemical Engineering
Consolidated
state nanoparticles are used as catalyst, electrodes in solar and fuel cells.
Bio-sensitive
nanoparticles are used in the production of DNA –chips, bio-sensors etc.
Nano-structed
ceramic materials are used in synthetic bones.
Few
nanomaterials are also used in adsorbents, self –cleaning glass, fuel
additives, drugs, ferrofluids, paints etc.
Nano
–metallic colloids are used as film precursors.
7 NON
LINEAR MATERIALS (NLO MATERIALS)
The change in optical properties due to electrical and magnetic
field associated with light is called non linear effects and those materials
which possess these effects are those materials possess these effects are
called non-linear materials.
Example:
Lithium
tantalate
Lithium
iodate (LiO3)
Barium
Sodum niobate
Ammonium-dihydrophosphate
(ADO)
Potassium-dyhydrophosphate
(KDP)
7.1
Higher Harmonic Generation
Higher (second) Harmonic generation represents the generation of
new frequencies with the help of the crystals such as quartz, LiO3,
etc.
Explanation
In a
linear medium, Polarization (P) is proportional to the electric field E that
induces it

When light of higher intensity is passed through dielectric medium,
the electric field has larges amplitude and the oscillation of dipoles are
distorted. Therefore, some nonlinearity is observed beteen P and E and hence
the higher fields are written as
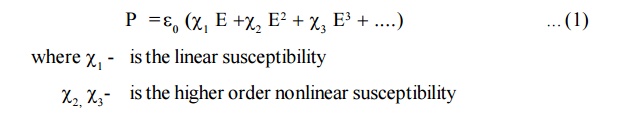
with increase of higher order terms come into play. Let us assume that the field is strong enough to give rise to χ2.
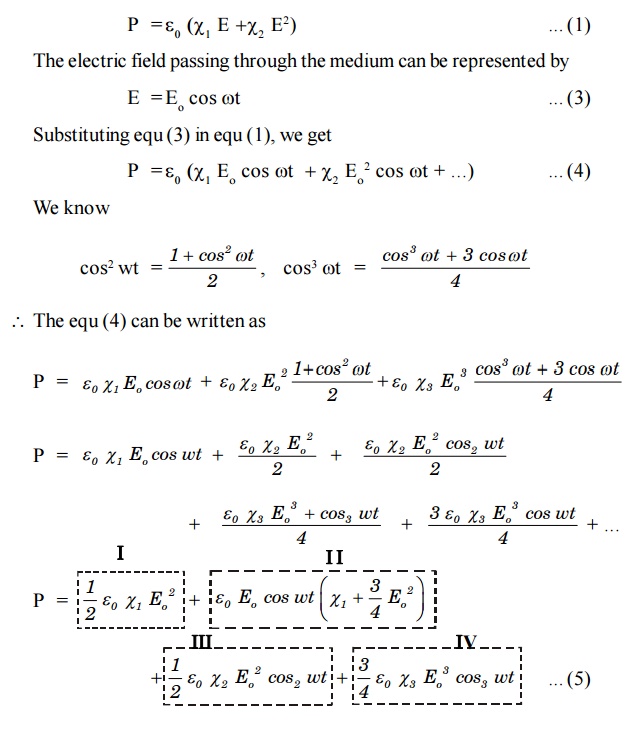
This non linear polarization shows that it contains the second
harmonic of (III term) as well as an average term (I term) called optical
rectification. It can be shown that only in the crystals lacking inversion
symmetry, second harmonic generation (SHG) is possible.
7.2
Experimental Proof
When the fundamental radiation from a laser is sent through SHG
crystal, due to SHG, conversion to double the frequency ie. half the wave
length takes place. For example 1.064 m radiation from Nd-YAG laser gets
converted to 0.532 m on passing through crystals like KDP, ADP, etc.
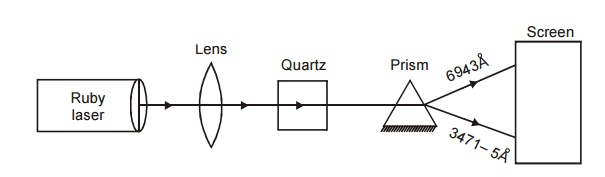
Fig. 5.7 Experiment
arrangement for SHG
If the incident radiation from the laser is intense enough such
that the polarization needs to be represented by three terms.
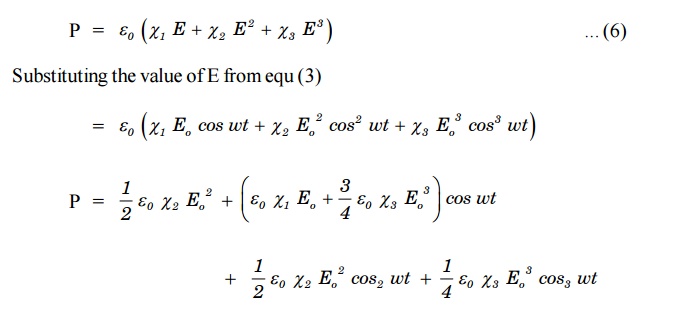
The last term in the above equation represents third harmonic generation at frequency 3ω . Likewise one can account for higher harmonic generation.
7.3
Optical mixing
We are familiar with ariving at a new colour by mixing of two
colours in paints using non linear phenomena, called optical mixing, generation
new frequencies are possible. Suppose two coherent waves of unequal frequencies
1 and 2 are traversing the material, then
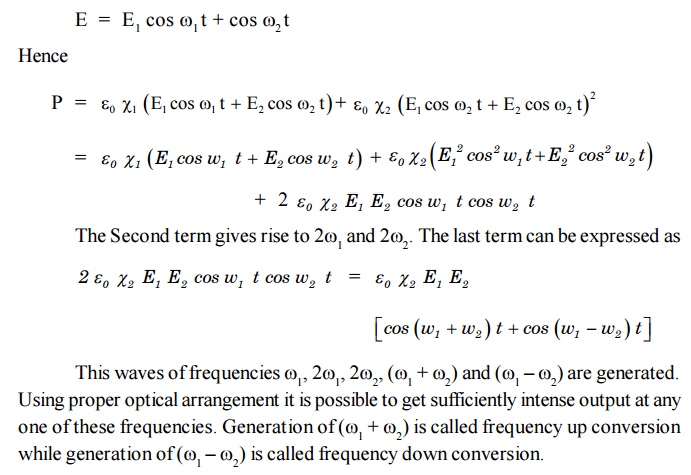
8
BIOMATERIALS
Biomaterials are used to make devices to replace a part or a
function of the body in safe, reliably economically, and physiologically
acceptable manner. A variety of devices and materials are used in the treatment
of disease or injury. Commonplace examples include suture needles, plates,
teeth fillings, etc. (or)
A biomaterial is a synthetic material used to replace part of a
living system or to function in intimate contact with living tissue of a human
body. Alone or as part of a complex system, is used to direct, by control of interactions
with components of living systems, the course of any therapeutic or diagnostic
procedure.
8.1
Biomaterials Classifications
When a synthetic material is placed within the human body, tissue
reacts towards the implant in a variety of ways depending on the material type.
The mechanism of tissue interaction depends on the tissue response to the
implant surface. In general, there are three terms in which a biomaterial may
be described in or classified into representing the tissues responses. These are
bioinert, bioresorbable, and bioactive.
1. Bioinert Biomaterials
The term bioinert refers to any material that once placed in the
human body has minimal interaction with its surrounding tissue. Examples of
these are stainless steel, titanium, alumina, partially stabilised zirconia,
and ultra high molecular weight polyethylene. Generally a fibrous capsule might
form around bioinert implants hence its bio functionality relies on tissue
integration through the implant.
2. Bioactive Biomaterials
Bioactive refers to a material, which upon being placed within the
human body interacts with the surrounding bone and in some cases, even soft
tissue. This occurs through a time - dependent kinetic modification of the
surface, triggered by their implantation within the living bone. An ion -
exchange reaction between the bioactive implant and surrounding body fluids -
results in the formation of a biologically active carbonate apatite (CHAp)
layer on the implant that is chemically and crystallographically equivalent to
the mineral phase in bone. Prime examples of these materials are synthetic
hydroxyapatite [Ca10 (PO4)6(OH)2],
glass ceramic A-W and bioglass.
3. Bioresorbable Biomaterials
Bioresorbable refers to a material that upon placement within the
human body starts to dissolve (resorbed) and slowly replaced by advancing
tissue (such as bone). Common examples of bioresorbable materials are
tricalcium phosphate [Ca3 (PO4)2] and
polylactic-polyglycolic acid copolymers. Calcium oxide, calcium carbonate and
gypsum are other common materials that have been utilised during the last three
decades.
8.2
Conventional implant devices
1. Ceramics
Inorganic compounds that contain metallic and non-metallic elements,
for which inter-atomic bonding is ionic or covalent, and which are generally
formed at high temperatures.
Example:
aluminum oxide, zirconia, calcium phosphates.
Uses of Ceramics
Structural
components
Joint
replacements, hip and knee
Spinal fusion
devices
Dental
crowns, bridges, implants, ect
Other
applications
Inner
ear and cochlear implants
Tissue
engineering
Coatings
for heart valves
Metals
Materials containing only metallic atoms either as single elements
or in combination in a closely packed crystal structure.
Example: stainless steel, cobalt alloys, titanium alloys.
Uses of Metals
Structural
components
Joint
replacements
Bone
fracture pins, plates
Dental
implants
Other
applications
Leads,
wires, tubing
Cardiac
devices
Polymers
From Greek - 'poly' meaning many and 'mer' meaning unit or part Low
density structures of non-metallic elements Often in the form of macromolecules
- chains, branched chains or cross linked networks Poor thermal and electrical
conductors due to the affinity of the elements to attract or share valence
electrons
Example:
Silicones, poly (ethylene), poly (vinyl
chloride), polyurethanes, polylactides
Uses of Polymers
Many
applications
Valves,
ducts, catheters
Joint
replacement
Coatings,
encapsulates
Tissue
engineering scaffolds
8.3 Biomaterials Properties
Mechanical
properties of the various tissues in the human body are well established, and
it is important that the in-vivo environment of an implant be considered during
implant design. Mechanical performance of an implanted device is influenced by
the inherent properties of the chosen biomaterial grades and by the processing
method used to convert them into their finished forms.
Degradation
properties of chosen biomaterials can play a pivotal role in determining tissue
healing dynamics and thus clinical outcome. Timescales involved in the tissue
regeneration process are increasingly well understood. Through transferring
functional requirement back to the native tissue during its regeneration,
clinically superior results may be achieved. Furthermore, resorption of the
implant can reduce both lifetime procedural cost and incidence of
post-operative complications.
Surface
properties of a biomaterial, determined by both its chemical composition and
conversion processes, affect the local tissue response at the
biomaterial-tissue interface on a cellular level. Consideration is therefore
given to the desired cellular response to a biomaterial surface, during medical
implant design.
8.4
Biomaterials Applications
Drug delivery
Cancer
detection, therapy and prevention.
Biomaterial-cell
interactions in applications such as tissue engineering, advanced stem-cell
culture, biosensors, and targeted drug delivery via the blood.
Advanced
drug delivery, ranging from polymeric aerosols that carry life-saving drugs to
cationic polymer/DNA self-assembled nanocomplexes that deliver DNA to specific
tissues for in vivo gene therapy.
Genetically-engineered
polymers for tissue engineering and drug delivery applications.
Cellular
biopolymers and their role in cell migration and cancer metastasis.
Other important applications
Heart-
pacemaker, artificial valve, artificial heart
Eye
-contact lens, intraocular lens
Ear
-artificial stapes, cochlea implant
Bone
-bone plate, intramedullary rod, joint prosthesis, bone cement, bone defect
repair
Kidney-
dialysis machine
Bladder-
catheter and stent
Muscle
sutures- muscle stimulator
Circulation-
artificial blood vessels
Skin
burn- dressings, artificial skin
Endocrine
-encapsulated pancreatic islet cells.
Related Topics