Chapter: Organic Chemistry: Acid–base reactions
Brønsted–Lowry acids and bases
BRØNSTED–LOWRY ACIDS AND BASES
Key Notes
Definition
The
Brønsted–Lowry definition of an acid is a molecule which can provide a proton.
The Brønsted–Lowry definition of a base is a molecule which can accept that
proton.
Brønsted–Lowry acids
A
hydrogen atom attached to an electronegative atom such as a halogen, oxygen, or
nitrogen is potentially acidic. Therefore, compounds containing the following
functional groups (carboxylic acid, phenol, alcohol, 1° and 2° amines,
and 1° and 2° amides) can act as Brønsted–Lowry acids.
Brønsted–Lowry bases
Examples
of Brønsted–Lowry bases include negatively charged ions and neutral molecules
containing oxygen or nitrogen (e.g. water, ethers, alco-hols, and amines).
Definition
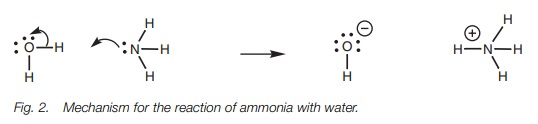
Put at its simplest, the Brønsted–Lowry definition of an acid is a molecule which can provide a proton. The Brønsted–Lowry definition of a base is a molecule which can accept that proton. An example of a simple acid/base reaction is the reaction of ammonia with water (Fig. 1). Here, water loses a proton and is an acid. Ammonia accepts that proton and is the base.

As far as the mechanism of the reaction is concerned,
the ammonia uses its lone pair of electrons to form a new bond to the proton
and is therefore acting as a nucleophile. This means that the water is acting
as an electrophile.
As the nitrogen uses its lone pair of electrons
to form the new bond, the bond between hydrogen and oxygen must break since
hydrogen is only allowed one bond. The electrons making up the O–H bond will
move onto oxygen to produce a third lone pair of electrons, thus giving the
oxygen a negative charge (Fig. 2).
Since the nitrogen atom on ammonia has used its lone pair of electrons to form
a new bond, it now has to share the electrons with hydrogen and so nitrogen
gains a positive charge.
Brønsted–Lowry acids
A Brønsted–Lowry acid is a molecule which contains an acidic hydrogen. In order to be acidic, the hydrogen must be slightly positive or electrophilic. This is possi-ble if hydrogen is attached to an electronegative atom such as a halogen, oxygen, or nitrogen. The following mineral acids and functional groups contain hydrogens which are potentially acidic (Fig. 3).
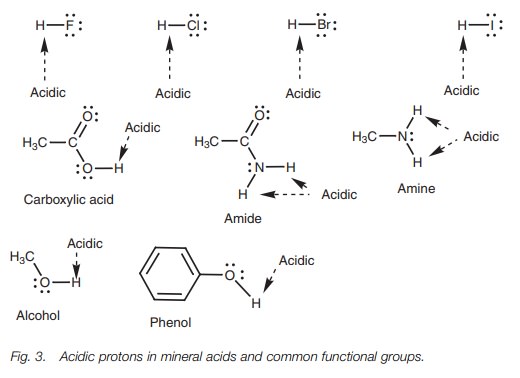
Hydrogens attached to carbon are not normally
acidic. However, we shall look at special cases where hydrogens attached to
carbon are acidic.
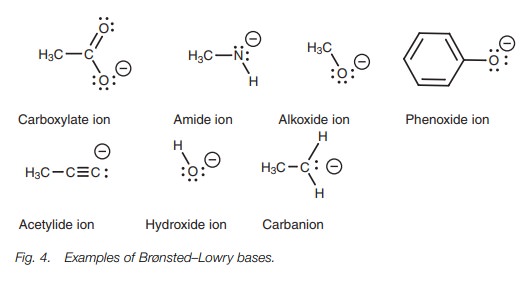
Brønsted–Lowry bases
A Brønsted–Lowry base is a molecule which can form a bond to a proton. Examples include negatively charged ions with a lone pair of electrons (Fig. 4).
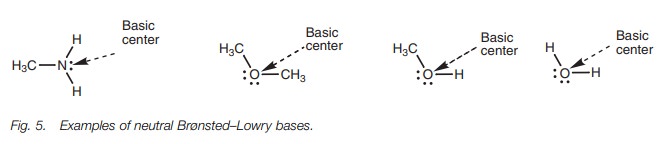
Neutral molecules can also act as bases if they
contain an oxygen or nitrogen atom. The most common examples are amines.
However, water, ethers and alco-hols are also capable of acting as bases (Fig. 5).
Related Topics