Chapter: Modern Pharmacology with Clinical Applications: Drugs for the Control of Supragingival Plaque
Antiplaque Agents
ANTIPLAQUE
AGENTS
Bisbiguanides
Chlorhexidine is a
symmetrical cationic molecule that is most stable as a salt; the highly
water-soluble diglu-conate is the most commonly used preparation. Be-cause of
its cationic properties, it binds strongly to hy-droxyapatite (the mineral
component of tooth enamel), the organic pellicle on the tooth surface, salivary
pro-teins, and bacteria. Much of the chlorhexidine binding in the mouth occurs
on the mucous membranes, such as the alveolar and gingival mucosa, from which
sites it is slowly released in active form.
Pharmacokinetics
The rate of clearance of chlorhexidine from the mouth after one mouth rinse with 10 mL of a 0.2% aqueous so-lution follows approximately first-order kinetics, with a half-life of 60 minutes. This means that following appli-cation of a single rinse with a 0.2% chlorhexidine solution, the concentration of the compound exceeds the minimum inhibitory concentration (MIC) for oral streptococci (5 mg/mL) for almost 5 hours. The pro-nounced substantivity, along with the relative suscepti-bility of oral streptococci, may account for the great ef-fectiveness of chlorhexidine in inhibiting supragingival plaque formation.
Mechanisms of Action
Although chlorhexidine
affects virtually all bacteria, gram-positive bacteria are more susceptible
than are gram-negative organisms. Furthermore, Streptococcus mutans and Antinomies viscosus seem to be
particularly sensitive. S. mutans has been associated with the
forma-tion of carious lesions in fissures and on interproximal tooth surfaces
and has been identified in large numbers in plaque and saliva samples of
subjects with high caries activity.
Low concentrations of chlorhexidine
are bacterio-static, while high concentrations are bactericidal. Bacteriostasis
is the result of chlorhexidine binding to the negatively charged bacterial cell
wall (e.g., lipo-polysaccharides), where it interferes with membrane transport
systems. Oral streptococci take up sugars via the phosphoenolpyruvate-mediated
phosphotrans-ferase (PEP-PTS) system. The PEP-PTS is a carrier-me-diated group
translocating process in which a number of soluble and membrane-bound enzymes
catalyze the transfer of the phosphoryl moiety of PEP to the sugar substrate
with the formation of sugar phosphate and pyruvate. Chlorhexidine is known to
abolish the activity of the PTS at bactericidal concentrations. High
chlor-hexidine concentrations cause intracellular protein pre-cipitation and
cell death. Despite its pronounced effect on plaque formation, no detectable
changes in resist-ance of plaque bacteria were found in a 6-month longi-tudinal
study of mouth rinses.
Clinical Uses
The previous routine
treatment for cases of severe gin-gival disease consisted of calculus and
plaque removal and oral hygiene instructions. Subsequent resolution of the
gingival inflammation was largely dependent on daily plaque control by the
patient. However, the use of a 0.1 to 0.2% chlorhexidine mouthwash
supplementing daily plaque control will facilitate the patient’s effort to
fight new plaque formation and to resolve gingivitis. Consequently, use of
chlorhexidine is indicated in the following situations: in disinfection of the
oral cavity be-fore dental treatment; as an adjunct during initial ther-apy,
especially in cases of local and general aggressive periodontitis; and in
handicapped patients.
Adverse Effects and Toxicity
The most conspicuous side
effect of chlorhexidine is the development of a yellow to brownish extrinsic
stain on the teeth and soft tissues of some patients. The discol-oration on
tooth surfaces is extremely tenacious, and a professional tooth cleaning using
abrasives is necessary to remove it completely. The staining is dose dependent,
and variation in severity is pronounced between indi-viduals. This side effect
is attributed to the cationic nature of the antiseptic. Desquamative soft
tissue lesions have also been reported with use of drug concentrations
exceeding 0.2% or after prolonged application. A fre-quently observed side
effect is impaired taste sensation. It was reported that rinsing with a 0.2%
aqueous solu-tion of chlorhexidine digluconate resulted in a signifi-cant and
selective change in taste perception for salt but not for sweet, bitter, and
sour.
In vitro, chlorhexidine can
adversely affect gingival fibroblast attachment to root surfaces. Furthermore,
protein production in human gingival fibroblasts is re-duced at chlorhexidine
concentrations that would not affect cell proliferation. Such findings
corroborate ear-lier studies showing delayed wound healing in stan-dardized
mucosal wounds after rinsing with 0.5% chlorhexidine solution.
As an oral rinsing agent, to
date chlorhexidine has not been reported to produce any toxic systemic effects.
Since chlorhexidine is poorly absorbed in the oral cav-ity and gastrointestinal
tract, little if any enters the bloodstream. A summary of chlorhexidine oral
rinses is given in Table 42.1.
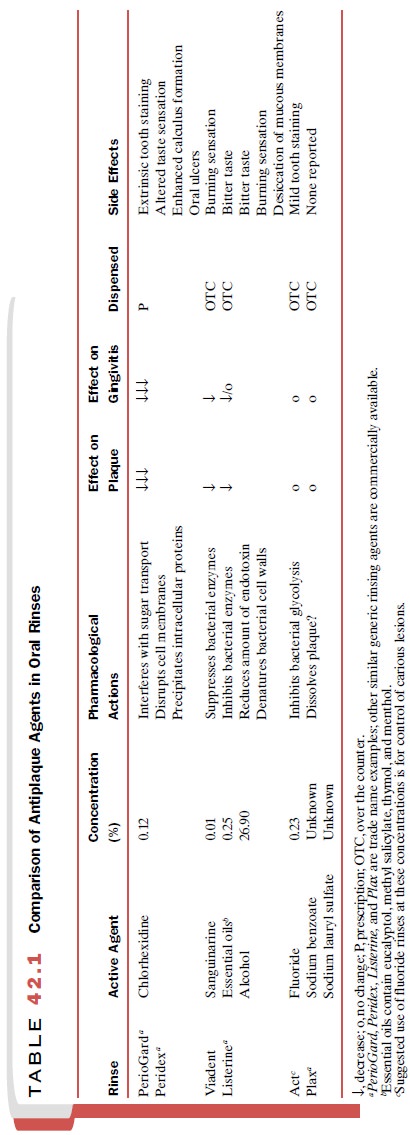
Nonionic Bisphenols
Triclosan is a broad-spectrum
antimicrobial compound. It was originally used in soaps, antiperspirants, and
cos-metic toiletries as a germicide. Today, triclosan is incor-porated into
toothpaste because of its wide spectrum of antimicrobial activities and low
toxicity.
Pharmacokinetics
Triclosan is retained in
dental plaque for at least 8 hours, which in addition to its broad
antibacterial prop-erty could make it suitable for use as an antiplaque agent
in oral care preparations. However, the com-pound is rapidly released from oral
tissues, resulting in relatively poor antiplaque properties as assessed in
clin-ical studies of plaque formation. This observation is fur-ther
corroborated by a poor correlation between mini-mal inhibitory concentration
values generated in vitro and clinical plaque inhibitory properties of
triclosan. Improvement of substantivity was accomplished by in-corporation of
triclosan in a polyvinyl methyl ether maleic acid copolymer (PVM/MA, Gantrez). With the combination of PVM/MA
copolymer and triclosan, the substantivity of the triclosan was increased to 12
hours in the oral cavity.
Mechanism of Action
Triclosan is active against a broad range of oral gram-positive and gram-negative bacteria. The primary target of its antibacterial activity is the bacterial cell mem-brane. High concentrations cause membrane leakage and ultimately lysis of the bacterial cell.
Effects at lower concentration are more
subtle. Triclosan has been shown to bind to cell membrane targets and inhibit
en-zymes associated with the phosphotransferase and pro-ton motive force
systems.
Clinical Effects
Triclosan plus copolymer is
available in toothpaste. Commercially available dentifrice concentrations
con-tain 0.3% triclosan and 2.0% PVM/MA copolymer. This product (Total) was tested in a large number of
short-term controlled clinical trials, from which a statis-tically significant
but clinically modest 15 to 20% plaque reduction was reported. The same
toothpaste composi-tion also exhibited significant anticalculus properties.
Typically, the reported reductions in calculus formation ranged from 25 to 35%.
Finally, of considerable interest is the observation that triclosan inhibits
gingivitis by a mechanism independent of its antiplaque activity. In a clinical
study, minimal plaque effects accompanied an average 50% reduction in
gingivitis. An explanation of this surprising effect stems from research
conducted us-ing a gingival fibroblast cell culture model. These exper-iments
revealed that triclosan could inhibit the IL-1-induced production of
prostaglandin E2.
Essential Oils
A mixture of essential oils
consisting of thymol 0.06%, eucalyptol 0.09%, methyl salicylate 0.06%, and
menthol 0.04% in an alcohol-based vehicle (26.9%) provides the
plaque-inhibiting properties of rinsing agents such as Listerine.
Essential oils may reduce
plaque levels by inhibiting bacterial enzymes and by reducing pathogenicity of
plaque via reduction of the amount of endotoxin; the al-cohol is probably
responsible for denaturing bacterial cell walls. The substantivity of Listerine
appears to be quite low, and therefore, it must be used at least twice a day to
be effective. A variety of clinical studies have demonstrated that Listerine is
capable of reducing plaque and gingivitis over extended periods; however, the
degree of reduction is variable. Listerine will reduce plaque and gingivitis
anywhere from 14.9 to 20.8% and 6.5 to 27.7%, respectively (Table 42.1).
Adverse reac-tions include a bitter taste and burning sensation in the oral
cavity. Regular use of high-alcohol rinses can ag-gravate existing oral lesions
and desiccate mucous membranes. In addition to Listerine, a huge number of
American Dental Society (ADA) approved generic equivalents available over the
counter.
Fluorides
Fluorides are widely used in
caries prevention, for which they have been highly effective. Systemic
administration of fluorides for caries prevention is available via drink-
ing water (1 mg/ L), tablets
(0.25–1 mg), drops (0.125–0.5 mg), topical application by mouthwashes
(200–1,000 mg/L), gels for home use (900 mg/kg) and professional use
(9,000–19,000 mg/kg), and dentifrices (1,000 mg/kg). In contrast to the
efficacy of fluorides in preventing car-ious lesions, these formulations have
relatively poor an-tibacterial properties (Table 42.1). The weak therapeutic
benefit of fluorides on gingivitis is due to a modest inhi-bition of glycolysis
in plaque bacteria. Sodium fluoride, monofluorophosphate, and stannous fluoride
are the compounds used in topically applied agents.
A few well-controlled
clinical studies suggested a potential plaque-inhibiting effect for dentifrices
con-taining stannous fluoride. However, these results were most likely due to
the stannous ion rather than to fluoride; the positive charge of the stannous
ion may in-terfere with bacterial membrane function, bacterial adhesion, and
glucose uptake, thereby inhibiting the formation of plaque.
Mild tooth staining has been
observed after use of stannous fluoride products. The ADA Council on Dental Therapeutics
endorses fluorides for their caries-inhibiting effect but not for plaque
inhibition.
Prebrushing Rinses
The topical application of a
liquid rinse before brushing as an aid in the mechanical removal of
supragingival plaque is a novel idea. Since the introduction of the first
prebrushing rinse there has been a rapid increase in the number of generic
products that claim to physically loosen or remove plaque. Prebrushing rinses
usually contain a plethora of ingredients, and it is not known which constituent
is the active chemical. It has been sug-gested that sodium lauryl sulfate acts
as a detergent to dislodge or loosen the plaque on teeth (Table 42.1). When
prebrushing rinses were tested against placebo rinses, prebrushing rinses
appeared to have no effect on plaque reduction.
Related Topics