Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Fishes as social animals: aggregation, aggression, and cooperation
Visual communication of Fishes - Fishes as social animals
Communication
Communication involves the transfer of information between individuals during which at least the signal sender derives some adaptive benefit (Myrberg 1981). To send information, the signal sender must contrast with (stand out from) the background. Although this is most obvious (to us) in a visual context – where bright objects are most easily seen against dark backgrounds and dark objects against bright backgrounds – the contrast principle applies to all sensory modes. Background noise, be it visual, acoustic, chemical, tactile, or electric, will mask a signal. Information is transmitted when the signal exceeds the noise; conversely, an animal becomes cryptic if it blends in with the background. The message sent usually results in repulsion or attraction or may inform the signal receiver about the physiological state or behavioral motivation of the sender. Frequently, signals from several modes are combined to enhance the message and reduce ambiguity.
Visual communication
Vision plays a critical role in fish communication in most environments. Coloration is dependent on hue (wavelength mixtures), saturation (wavelength purity), andbrightness (light intensity) (Hailman 1977; Levine et al. 1980). Coloration is incorporated into scales, skin, fins, and eyes as the product of pigments, achromatic elements, or structural colors. Pigmented cells (chromatophores) in the dermis contain carotenoids and other compounds and reflect yellow, orange, and red. Achromatics are black and white. Black coloration results from the movement of melanin granules within melanophores; dispersed melanin darkens a fish, whereas melanin concentrated in the melanophores makes the fish appear lighter in color. White coloration comes from light reflected by guanine crystals in leucophores and iridophores. Greens, blues, and violets are generally structural colors produced by light refracted and reflected by layers of skin and scales; the color depends on the thickness of the layers relative to the wavelength of the light (Lythgoe 1979; Levine et al. 1980).
The diversity of color in fishes is essentially unlimited, ranging from uniformly dark black or red in many deepsea forms, to silvery in pelagic and water column fishes, to countershaded in nearshore fishes of most littoral communities, to the strikingly contrasted colors of tropical freshwater and marine fishes. Visibility (and invisibility) depends on a combination of fish color, the transmission qualities of water in specific habitats, background characteristics, and the visual physiology of the eye, especially the retina (Losey et al. 2003; Marshall et al. 2003a, 2003b).
Melanin and guanine reflect light across the entire visible spectrum and are therefore “available” for use in almost all habitats. Black and white are among the most commonly used colors in fishes (e.g., minnows, characins, catfishes, sunfishes, damselfishes, butterfl yfishes, grunts, drums, cichlids, gobies, triggerfishes). In the clear waters of a coral reef or tropical lake, yellow and its complement indigo blue are most visible; these are the colors commonly found on butterfl y yfishes, angelfishes, grunts, damselfishes, parrotfishes, and wrasses on reefs and on characins, minnows, Guppies, rainbowfishes, and cichlids in tropical waters. Nearshore temperate habitats, particularly in fresh water, tend to be stained with organic compounds that give them a yellowish tinge. Red and its complement blue-green are more visible under these conditions and it is not sur prising that the breeding colors of minnows, salmonids, sticklebacks, darters, and sunfishes often incorporate these (Lythgoe 1979).
Colors on a fish’s body may be used in static or dynamic displays. Static coloration is generally an identification badge that informs about the species, sex, reproductive condition, or age of a fish. Species identification is achieved through a combination of body form and color; ichthyologists as well as fishes use this combination in determining a species’ identification (Thresher 1976). In the myctophid lanternfishes, the number and pattern of photophores (light organs) is species specific and probably aids in schooling and as a sexual isolating mechanism. The taxonomic skills of many fishes are quite good; the Beau Gregory Damselfish can apparently distinguish among approximately 50 different species of reef fishes that intrude on its territory (Ebersole 1977).
Sexual dimorphism in coloration and body morphology is common in fishes, occurring as a permanent distinction in many tropical species or more seasonally in temperate fishes; generally males are the dimorphic or more distinctive sex. Ontogenetically distinctive coloration may aid in the identification of potential schoolmates, augmenting the tendency of fishes to aggregate with members of equal size (see below). In at least 18 coral reef families, juveniles differ from adults in color pattern (Thresher 1984). As French grunts (Haemulidae) settle from the plankton and take up residence on a coral reef, they develop at least four distinctive color phases associated with changes in habitat and behavior (McFarland 1980).
Dynamic displays involve either rapid exposure of colored, previously hidden structures or changes in color. Dynamic displays include movements of the body, fins, operculae, and mouth. Often fin erection or gill-cover flaring exposes patches of color that contrast sharply with surrounding structures. Grunts open their mouths in headto- head encounters to expose a bright red mouth lining. Many fishes flare their gill covers during aggressive, head-on encounters; gills and gill margins often contrast with the rest of the body (salmonids, centrarchid sunfishes, cichlids, labrids, Siamese Fighting Fishes). Fin erection and coloration play a dominant role in visual displays, probably because the movement associated with their erection is particularly eye catching. As a result, differential coloration of fins (inclusion of spots and stripes) is common. Fin flicking serves in calling young to parents, as a schooling signal, and during agonistic interactions (“agonistic” refers to aggressive and submissive activities, as in the verb “to agonize”).
A special case of dynamic display involves the flashing of bacterially produced light by ponyfishes (Leiiognathidae), in which males “shine” their light inward toward a reflective coating of the swim bladder (Fig. 22.1). The light then passes outward through transparent skin and a moveable, muscular shutter in the body wall. Males in schools
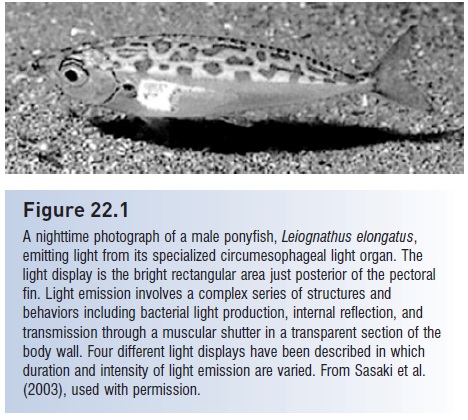
Figure 22.1
A nighttime photograph of a male ponyfish, Leiognathus elongatus, emitting light from its specialized circumesophageal light organ. The light display is the bright rectangular area just posterior of the pectoral fin. Light emission involves a complex series of structures and behaviors including bacterial light production, internal reflection, and transmission through a muscular shutter in a transparent section of the body wall. Four different light displays have been described in which duration and intensity of light emission are varied. From Sasaki et al. (2003), used with permission.
Changeable colors serve primarily to advertise alterations in the behavioral state of a fish, or to conceal a fish from aggressors or predators. During agonistic, predator– prey, and breeding interactions, individuals will blanch or darken and develop bars or spots on a moment to moment basis (minnows, dolphinfishes, rudderfishes, cichlids, damselfishes, surgeonfishes, tunas, flatfishes). One can often predict the winner of a territorial encounter by observing differences in body shading. On a circadian basis, even the most colorful fishes by day turn relatively dull or blotchy at night. For example, neon and cardinal tetras (Paracheirodon, Characidae), which are brilliant blue-green and red by day, assume an inconspicuous pinkish tinge as they rest on the bottom at night (Lythgoe & Shand 1983). Such changes suggest that many visually mediated agonistic interactions cease with nightfall, but that many piscivorous fishes are still capable of locating prey at night using visual cues (Helfman 1993).
Short-term color change is primarily under the immediate control of the nervous system, whereas longer term ontogenetic and seasonal changes are more likely controlled by hormone levels. Seasonal color change is most often associated with the onset of breeding activity, when territorial males develop bright, contrasting coloration. In the spring, North American minnows and darters assume color patterns that rival those in any tropical reef or river assemblage. Females in many of these species undergo less dramatic seasonal changes. Interesting ontogenetic changes occur in migratory salmonids and anguillids. Many juvenile salmonids live in streams and combine countershading with vertically oblong, dark “parr” marks that may be disruptive in function. These fish migrate to the open ocean as smolts and develop a silvery coloration that is more effective camoufl age in open water, pelagic situations. Upon returning to their natal (birth) stream, many species assume a bright, boldly contrasting breeding coloration that is the antithesis of camoufl age. Anguillid eels
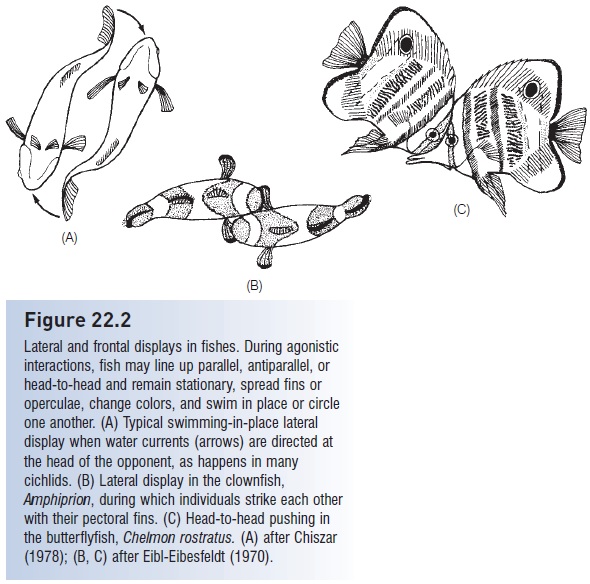
Figure 22.2
Lateral and frontal displays in fishes. During agonistic interactions, fish may line up parallel, antiparallel, or head-to-head and remain stationary, spread fins or operculae, change colors, and swim in place or circle one another. (A) Typical swimming-in-place lateral display when water currents (arrows) are directed at the head of the opponent, as happens in many cichlids. (B) Lateral display in the clownfish, Amphiprion, during which individuals strike each other with their pectoral fins. (C) Head-to-head pushing in the butterfl yfish, Chelmon rostratus. (A) after Chiszar (1978); (B, C) after Eibl-Eibesfeldt (1970).
Visual agonistic displays often involve highly stereotyped movements. Combat may involve lateral displays, where two fish swim in place with fins spread, oriented either parallel or antiparallel (head to tail) (Fig. 22.2). As an interaction escalates, fish may begin body beating, a vigorous swimming-in-place that pushes water at an opponent and that may indicate the relative strengths of the combatants. Hence tactile and acoustic, near-field information may be added to the visual display. Antiparallel fish may strike one another with the pectoral fins (as in the anemonefish, Amphiprion) or may “carousel”, swimming in tight circles around one another. Carouseling can lead to biting of caudal fins or chasing. Color changes frequently accompany lateral displays, and “color fi ghts” occur in some species as different color phases indicate different levels of aggression (e.g., the nandid, Badis badis; Barlow 1963). Frontal displays, sometimes with fish facing each other head on and even grabbing each other’s mouth, are also common (e.g., in grunts, Corkwing Wrasse, Kissing Gouramis).
Ritualized combat can decide the outcome of an interaction without actual physical fi ghting. It is in the best interests of both opponents to settle a dispute without incurring injury. The potential for such injury obviously varies among species, but can be considerable, as has been discovered by scuba divers who ignored the distinctive, ritualized, head-swinging displays of apparently territorial Gray Reef Sharks (Johnson & Nelson 1973) (Fig. 22.3). White Sharks also engage in apparently ritualized, agonistic displays toward other White Sharks, including parallel swimming and slapping the tail on the surface in the direction of another White Shark during a feeding bout (Klimley et al. 1996).
A particularly nice example of the multiple functions of visual displays involves the Flashlight Fish, Photoblepharon palpebratus (Anomalopidae; Morin et al. 1975). This 6 cm long, nocturnally active fish lives in the shallow waters of
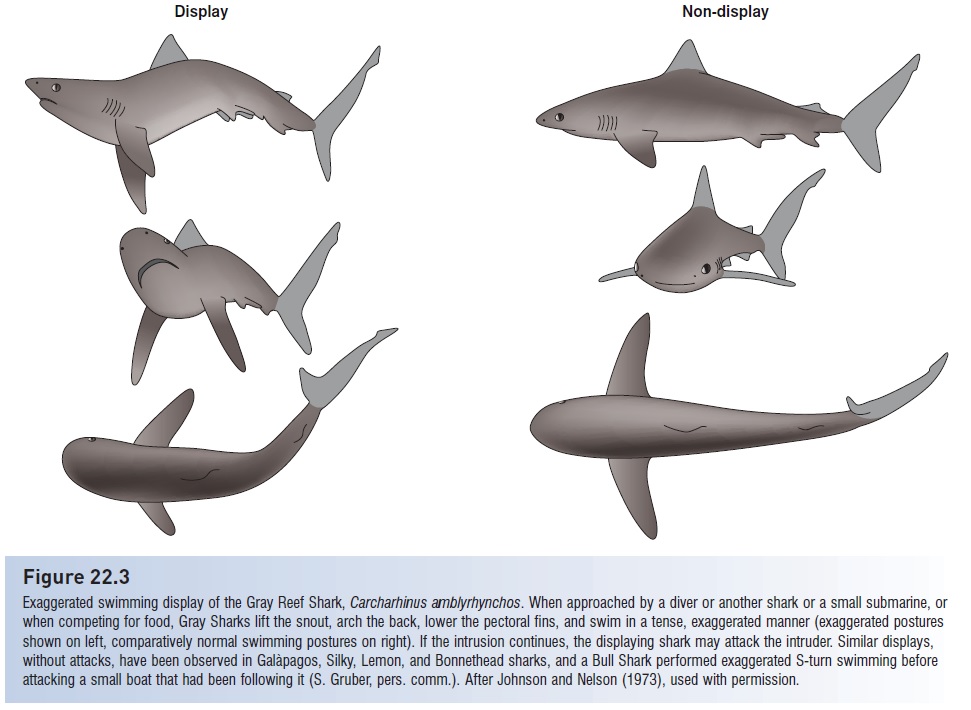
Figure 22.3
Exaggerated swimming display of the Gray Reef Shark, Carcharhinus amblyrhynchos. When approached by a diver or another shark or a small submarine, or when competing for food, Gray Sharks lift the snout, arch the back, lower the pectoral fins, and swim in a tense, exaggerated manner (exaggerated postures shown on left, comparatively normal swimming postures on right). If the intrusion continues, the displaying shark may attack the intruder. Similar displays, without attacks, have been observed in Galàpagos, Silky, Lemon, and Bonnethead sharks, and a Bull Shark performed exaggerated S-turn swimming before attacking a small boat that had been following it (S. Gruber, pers. comm.). After Johnson and Nelson (1973), used with permission.
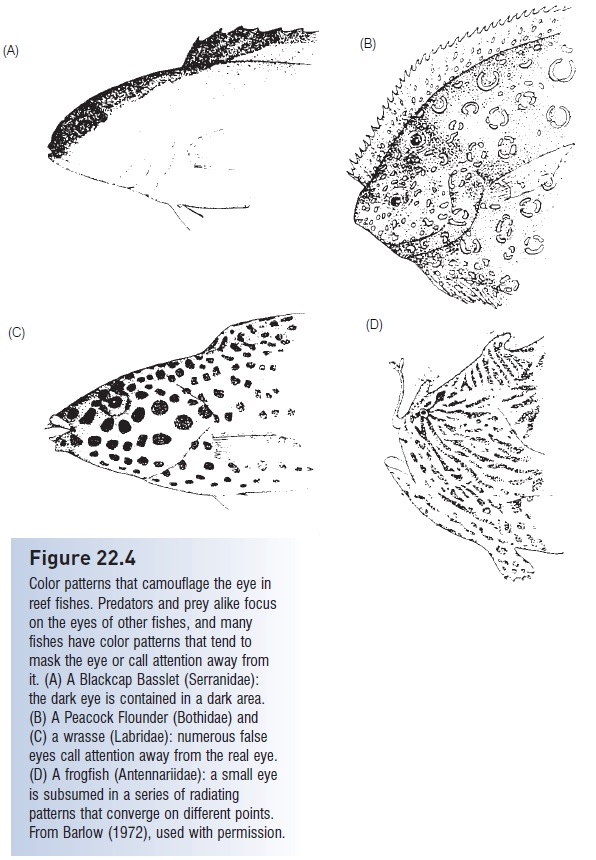
Figure 22.4
Color patterns that camouflage the eye in reef fishes. Predators and prey alike focus on the eyes of other fishes, and many fishes have color patterns that tend to mask the eye or call attention away from it. (A) A Blackcap Basslet (Serranidae): the dark eye is contained in a dark area. (B) A Peacock Flounder (Bothidae) and (C) a wrasse (Labridae): numerous false eyes call attention away from the real eye. (D) A frogfish (Antennariidae): a small eye is subsumed in a series of radiating patterns that converge on different points. From Barlow (1972), used with permission.

Figure 22.5
A Fourspot Butterfl yfish, Chaetodon quadrimaculatus, in the field that has suffered an obvious wound in the region of its posterior ocellus or eye spot. Rare photos such as this one are indirect evidence that ocelli serve as deflective marks that direct the attacks of predators away from more vulnerable head regions. Photo by P. Motta, used with permission.
It possesses a semicircular luminous organ just below each eye that contains continuously emitting bioluminescent bacteria. The light can be turned on and off by means of a muscular lid. The Flashlight Fish is unique in that it forms shoals at night and uses its light for feeding, predator avoidance, and in behavioral interactions. The light is turned on to attract zooplankton prey and then to illuminate prey. If approached by a predator, the Flashlight Fish swims with the light on and then turns it off and changes direction. The fish thus moves to a place that was unpredictable from its former direction of movement. In a social context, shoals form at night when small groups swim close enough to see each other’s lights. Male–female pairs hold territories over the reef. If an intruding Photoblepharon approaches, the female swims up to it with her light off and then turns the light on literally in the face of the intruder, causing it to depart (Morin et al. 1975).
A final category of visual signals and reception has caught the attention of fish biologists in just the past decade.
This is the topic of ultraviolet (UV) reflectance and detection (Siebeck et al. 2006). The spectrum of human-visible light falls between 400 and 700 nm, perceived as violet to red colors. UV-A radiation lies between 320 and 400 nm and is invisible to humans. However, many marine as well as freshwater fishes, from elasmobranchs to higher teleosts, have eyes that do not block UV light and that possess retinal pigments with maximal absorption characteristics well in the UV range (Losey et al. 2003). Hence “many teleost fishes may be adapted for vision in the UV range” (Losey et al. 1999a, p. 921).
Although UV light is scattered rapidly in water, biologically useful amounts of UV light penetrate clear aquatic environments to at least 100 m depth. UV light can be especially useful for detecting zooplankton against an open water background (e.g., Jordan et al. 2004). In a social context, the rapid scattering of UV light means that skin pigments that reflect UV, which have been found on the fins, head, and bodies in at least 21 families of reef fishes, will be visible only over short distances. This creates an ideal condition for social signaling at short range while minimizing eavesdropping by other species such as predators (e.g., Cummings et al. 2003; Losey 2003). UV reflection and detection is increasingly proving to play a role in fish social behavior, including mate choice in Guppies and Three-spined Sticklebacks (Smith et al. 2002; Rick et al. 2006), shoaling decisions in sticklebacks (Modarressie et al. 2006), and territorial encounters in damselfishes (Siebeck 2004). UV detection may be important in the ability of fishes to detect polarized light, providing additional opportunities for target discrimination in foraging and social signaling as well as affecting orientation ability (Mussi et al. 2005). The visual world of fishes may be very different from ours, and our attempts at interpreting visual and other signals require capitalizing on developing technologies and keeping an open mind.
Related Topics