Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Economic Considerations in Medical Biotechnology
The Value of a New Medical Technology
THE VALUE OF A NEW MEDICAL TECHNOLOGY
The task of determining the value of a new agent should fall somewhere
within the purview of the marketing function of a firm. Although some
compa-nies have established health care economic capabil-ities within the
clinical research structure of their organizations, it is essential that the
group that addresses the value of a new product does so from the perspective of
the market and not of the company or the research team. This is important for
two reasons. First, evaluating the product candidate from the perspective of
the user, and not from the team that is developing it, can minimize the bias
that is inherent in evaluating one’s own creations. Second, and most
importantly, a market focus will move the evaluation away from the technical
and scientifically interesting aspects of the product under evaluation and
toward the real utility the product might bring to the medical care
marketplace. Although the scientific, or purely clinical, aspects of a new
product should not be discounted, when the time comes to measure the economic contribution
of a new agent, those devel-oping the new agent must move past these
considera-tions. It is the tangible effects that a new treatment will have on
the patient and the health care system that determine its value, not the
technology support-ing it. The phrase to keep in mind is “value in use.”
The importance of a marketing focus when evaluating the economic effects
of a new agent, or product candidate, cannot be overstated. Failing to consider
the product’s value in use can result in overly optimistic expectations of
sales performance and market acceptance. Marketing is often defined as the
process of identifying and filling the needs of the market. If this is the
case, then the developers of new pharmaceutical technologies must ask two
questions: “What does the market need?” and “What does the market want?”
Analysis of the pharmaceutical market in the first decade of the twenty first
century will show that the market needs and wants:
n Lower costs
n Controllable costs n Predictable cost
n Improved outcomes
Note that this list does not include new therapeutic agents. From the
perspective of many payers, authorities, clinicians, and buyers, a new agent,
in and of itself, is a problem. The effort required to evaluate a new agent and
prepare recommendations to adopt or reject it takes time away from other
efforts. For many in the health care delivery system a new drug means more
work—not that they are opposed to innovation, but newness in and of itself,
regardless of the technology behind it, has no intrinsic value. The value of
new technologies is in their efficiency, their ability to render results that
are not available through other methods or at costs significantly lower than
other interventions. Documenting and understanding the economic effects of new
technologies on the various health care systems helps the firm to allocate its
resources more appropriately, accelerate the adoption of new tech-nologies into
the health care system, and reap the financial rewards of its innovation.
There are many different aspects of the term “value,” depending upon the
perspective of the individual or group evaluating a new product and the needs
that are met by the product itself. When developing new medical technologies,
it is useful to look to the market to determine the aspects of a product that
could create and capture the greatest amount of value. Two products that have
entered the market in recent years provide good examples of the different ways
in which value is assessed.
Activase [tissue plasminogen activator (tPA)] from Genentech, one of the
first biotechnology entrants in health care, entered the market priced at
nearly ten times the price level of streptokinase, its nearest competitor. This
product, which is used solely in the hospital setting, significantly increased
the cost of medical treatment of patients suffering myocardial infarctions. But
the problems associated with strepto-kinase and the great urgency of need for
treatments for acute infarctions were such that many cardiolo-gists believed
that any product that proved useful in this area would be worth the added cost.
The hospitals, which in the United States are reimbursed on a capitated basis
for the bulk of such procedures, were essentially forced to subsidize the use
of the agent, as they were unable to pass the added cost of tPA to many of
their patients’ insurers. The pricing of the product created a significant
controversy, but the sales of Activase and its successors have been growing
consistently since its launch. The key driver of value for tPA has been, and
continues to be, the urgency of the underlying condition. The ability of the
product to reduce the rate of immediate mortality is what drives its value.
Once the product became a standard of care, incidentally, reimbursement rates
were increased to accommodate it, making its economic value positive to
hospitals.
A product that delivered a different type of value is the
colony-stimulating factor from Amgen (G-CSF, Neupogen ). Neupogen was priced
well below its economic value. The product’s primary benefit is in the
reduction of serious infections in cancer patients, who often suffer
significant decreases in white blood cells due to chemotherapy. By bolstering
the white count, Neupogen allows oncol-ogists to use more efficacious doses of
cytotoxic oncology agents while decreasing the rate of infection and subsequent
hospitalization for cancer patients. It has been estimated that the use of
Neupogen reduces the expected cost of treating infections by roughly $6,000 per
cancer patient per course of therapy. At a price of roughly $1400 per course of
therapy, Neupogen not only provides better clinical care but also offers
savings of approximately $4600 per patient. The economic benefits of the
product have helped it to gain use rapidly with significantly fewer
restrictions than products such as tPA, whose economic value is not as readily
apparent.
These two very successful products provide clear clinical benefits, but
their sources of value are quite different. The value of a new product may come
from several sources, depending on the needs of clinicians and their
perceptions of the situations in which they treat patients. Some current
treatments bring risk, either because of the uncertainty of their effects on
the patient (positive or negative) or because of the effort or cost required to
use or understand the treatments. A new product that reduces this risk will be
perceived as bringing new value to the market. In such cases, the new product
removes or reduces some negative aspects of treatment. Neupogen, by reducing
the chance of infection and reducing the average cost of treatment, brought new
value to the marketplace in this manner.
Value can come from the enhancement of the positive aspects of treatment
as well. A product that has a higher rate of efficacy than current therapies is
the most obvious example of such a case. But any product that provides benefits
in an area of critical need, where few or no current treatments are available,
will be seen as providing immediate value. This was, and remains, the case for
tPA.
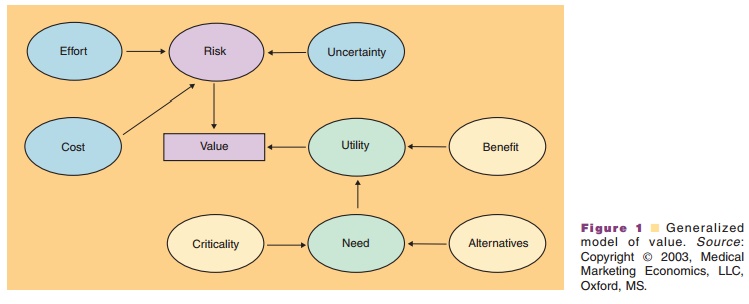
Any new product under development should be evaluated with these aspects
of value in mind. A generalized model of value, presented in Figure 1, can be
used to determine the areas of greatest need in the marketplace for a new agent
and to provide guidance in product development. By talking with clinicians,
patients, and others involved in current treatments and keeping this model in
mind, the shortcomings of those current approaches can be evaluated and the
sources of new incremental value can be determined.
Understanding the source of the value brought to the market by a new
product is crucial to the development of the eventual marketing strategy. Using
Figure 1 as a guide, the potential sources of value can be determined for a
product candidate and appropriate studies, both clinical and economic, can be
designed to measure and demonstrate that value.
Related Topics