Chapter: Modern Pharmacology with Clinical Applications: The Renin–Angiotensin– Aldosterone System and Other Vasoactive Substances
The Renin–Angiotensin System
THE RENIN–ANGIOTENSIN
SYSTEM
The renin–angiotensin system
is important for the regulation of vascular smooth muscle tone, fluid and
electrolyte balance, and the growth of cardiac and vas-cular smooth muscle. A
normally functioning renin– angiotensin system contributes to the routine
control of arterial blood pressure. A variety of basic and clinical
investigations have resulted in a broader understanding of the role of the
renin–angiotensin system in the car-diovascular pathophysiology of
hypertension, conges-tive heart failure, and more recently, atherosclerosis.
Whether or not abnormal activity of the renin– angiotensin system contributes
to the primary etiology of these diseases, pharmacological
inhibition of the renin–angiotensin
system has proved to be a valuable therapeutic strategy in the treatment of
hypertension and congestive heart failure.
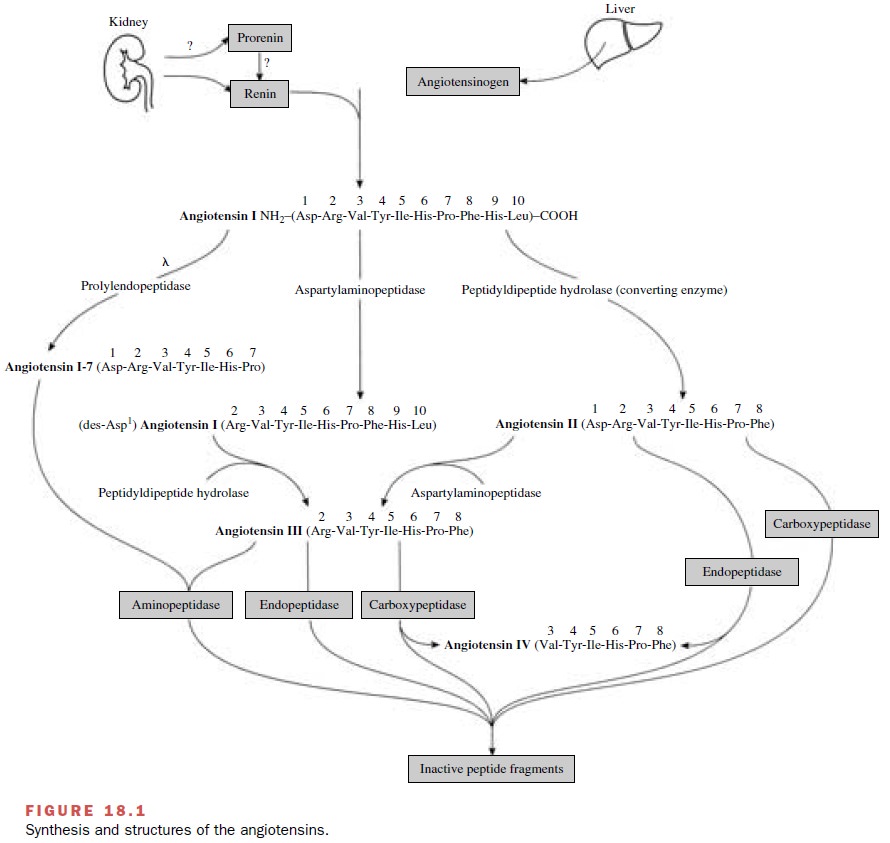
The classical
renin–angiotensin system comprises a series of biochemical steps (Fig. 18.1)
leading to the pro-duction of a family of structurally related peptides (e.g.,
angiotensin II, angiotensin III, and other smaller pep-tides with bioactivity).
Sites for pharmacological inter-vention in this system include the enzymatic
steps cat-alyzed by renin, angiotensin-converting enzyme (ACE), and angiotensin
receptors that mediate a particular physiological response.
Renin
Renin is an enzyme that is
synthesized and stored in the renal juxtaglomerular apparatus and that
catalyzes the formation of a decapeptide, angiotensin
I, from a plasma protein substrate. Renin has a narrow substrate
specificity that is limited to a single peptide bond in an-giotensinogen, a
precursor of angiotensin I. Renin is considered to control the rate-limiting
step in the ulti-mate production of angiotensin II. Control of renin se-cretion
by the juxtaglomerular apparatus is important in determining the plasma renin
concentration.
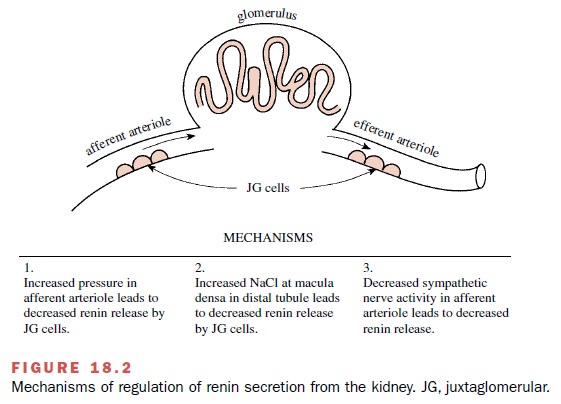
Three generally accepted
mechanisms are involved in the regulation of renin secretion (Fig. 18.2). The
first depends on renal afferent arterioles that act as stretch receptors or
baroreceptors. Increased intravascular pressure and increased volume in the
afferent arteriole inhibits the release of renin. The second mechanism is the
result of changes in the amount of filtered sodium that reaches the macula
densa of the distal tubule. Plasma renin activity correlates inversely with
dietary sodium intake. The third renin secretory control mech-anism is
neurogenic and involves the dense sympathetic innervation of the
juxtaglomerular cells in the afferent arteriole; renin release is increased
following activation of α1-adrenoceptors by the neurotransmitter norepi-nephrine.
Angiotensin II, the primary end product of the renin–angiotensin system, acts on the juxtaglomerular cells to inhibit the release of renin; this process is there-fore a negative feedback mechanism. The half-life of renin in the circulation is 10 to 30 minutes, with inacti-vation occurring primarily in the liver. Small amounts of renin are eliminated by the kidneys. Pure human rennin has been used to develop specific inhibitors of the en-zyme. Low-molecular-weight orally effective renin in-hibitors are under development.
Angiotensinogen
Human plasma contains a
glycoprotein called an-giotensinogen, which
serves as the only known substrate for
renin. Angiotensinogen must undergo proteolysis before active portions of the
protein are sufficiently un-masked to exert biological effects. Angiotensinogen
is synthesized in many organs, including the liver, brain, kidney, and fat. Its
gene transcription and plasma con-centrations increase following treatment with
adreno-corticotropic hormone (ACTH), glucocorticoids, thy-roid hormone, and
estrogens, as well as during pregnancy and inflammation and after nephrectomy.
Angiotensinogen also has been found in large quanti-ties in cerebrospinal and
amniotic fluid. Mutations in the angiotensinogen gene have been reported to be
linked to human hypertension.
Angiotensin-Converting Enzyme: A Peptidyl Dipeptide Hydrolase
Metabolism of angiotensinogen
by renin produces the decapeptide angiotensin
I. This relatively inactive pep-tide is acted on by a
dipeptidase-converting enzyme to produce the very active octapeptide angiotensin II. In addition to
converting enzyme, angiotensin I can be acted on by prolyl endopeptidase, an
enzyme that re-moves the first amino acid to form angiotensin 1-7, a peptide
primarily active in the brain. ACE has been identified in vascular endothelial
cells, epithelial cells of the proximal tubule and small intestine, male
germinal cells, and the central nervous system. The lung vascular endothelium
contains the highest concentration of ACE, and therefore, the lung serves as
the major organ for the production of circulating angiotensin II. Although ACE
was originally thought to be specific for the conversion of angiotensin I to
II, it is now known to be a rather nonspecific peptidyl dipeptide hydrolase
that can cleave dipeptides from the carboxy terminus of a number of endogenous
peptides (e.g., substance P, bradykinin). Peptides with penultimate prolyl
residues are not cleaved by converting enzyme; this accounts for the biological
stability of angiotensin II. Inhibition of converting enzyme results in an
elevated pool of an-giotensin I. A mutation deletion in the ACE gene has been
linked to a higher risk factor for hypertension, left ventricular hypertrophy,
and myocardial infarction.
The Angiotensins
The amino acid composition of
the peptides and en-zymes involved in the synthesis and metabolism of the
angiotensins is shown in Figure 18.1. Angiotensin I is be-lieved to have little
direct biological activity and must be converted to angiotensin II or angiotensin
1-7 before characteristic responses of the renin–angiotensin system are
manifested. Angiotensin I and II are metabolized at their animo terminus by aspartyl aminopeptidase, an en-zyme in
plasma and numerous tissues. Angiotensin II is rapidly metabolized by aspartyl
aminopeptidases, en-dopeptidases, and carboxypeptidases, while angiotensin III
is hydrolyzed by aminopeptidases, endopeptidases, and carboxypeptidases (Fig.
18.1). The biological activity of angiotensin III ranges from one-fourth to equipotent
with angiotensin II, depending on the response being monitored. The smallest
biologically active peptide in this system is angiotensin IV, which exerts
unique ac-tions in the central nervous system and periphery that are distinct
from those of angiotensin II.
Related Topics