Chapter: Pathology: Hematopoetic Pathology–White Blood Cell Disorders & Lymphoid and Myeloid Neoplasms
Myeloid Neoplasms
MYELOID NEOPLASMS
Acute Myelogenous Leukemia
Acute myelogenous leukemia is
a cancer of the myeloid line of blood cells. Median age at diagnosis is age 50.
Symptoms include fatigue, unusual bleeding, and infec-tions.
Lab findings: Myeloid blasts
or promyelocytes represent at least 20% of the marrow cells. Auer rods (linear condensations of
cytoplasmic granules) are characteristic of AML and are not found in normal
myeloid precursors.
The WHO classification of AML
(2008) is as follows:
•
AML with recurrent genetic abnormalities (for some of these
entities, the karyotype is diagnostic regardless of the blast percentage)
o Promyelocytic leukemia has
t(15;17)(q22;q12) with fusion gene PML/RARA and responds to all-transretinoic
acid (ATRA); DIC is common
o AML with either
t(8;21)(q22;q22) or inv(16)(p13.1;q22)
•
AML with myelodysplasia-related changes
•
Therapy-related myeloid neoplasms
•
AML, not otherwise specified
Myelodysplastic Syndromes (MDS)
MDS are classified according
to the number of blasts in the marrow. Dysplastic changes include Pelger-Huët
cells (“aviator glasses” nuclei), ring sideroblasts, nuclear budding, and “pawn
ball” megakaryocytes. MDS are considered preleukemias, so patients are at
increased risk for developing acute leukemia.
MDS mainly affect older
adults (age 50–70); they also predispose to infection, hem-orrhage and anemia.
Transformation to AML is common.
Myeloproliferative Neoplasms (MPN)
MPN are clonal neoplastic
proliferations of multipotent myeloid stem cells. The bone marrow is usually
markedly hypercellular (hence the name myeloproliferative).
All cell lines are increased in number (erythroid, myeloid, and
megakaryocytes).
Chronic myelogenous leukemia (CML) is a clonal proliferation of
pluripotent granulocytic precursor stem
cells. In most cases it is associated with a BCR-ABL fusion gene due to a balanced (9;22) translocation;
however, this Phila-delphia chromosome is not specific to CML.
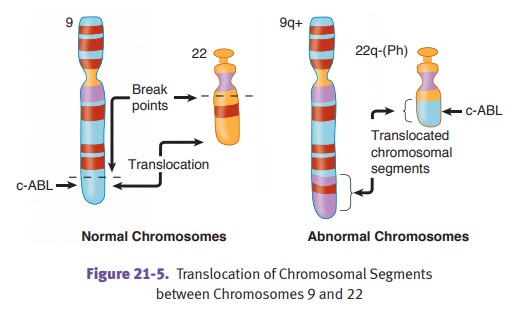
CML has an insidious onset (i.e., chronic) and
causes massive spleno-megaly. Progression is typically slow (50% develop
accelerated phase <5 years), unless blast crisis develops (very poor
prognosis; doesn’t respond to chemotherapy). In blast crisis, 70% of cases show
myeloid blasts and 30% show lymphoid blasts.
Microscopically, the bone marrow is
hypercellular, with all cell lines increased in number. Peripheral leukocytosis
is present, including mark-edly increased neutrophils (and bands and
metamyelocytes), as well as increased eosinophils and basophils (as in the
other MPS).
Treatment is imatinib mesylate, which blocks
the P210 tyrosine kinase protein produced by the translocation. Hematopoietic
stem cell trans-plantation is also used.
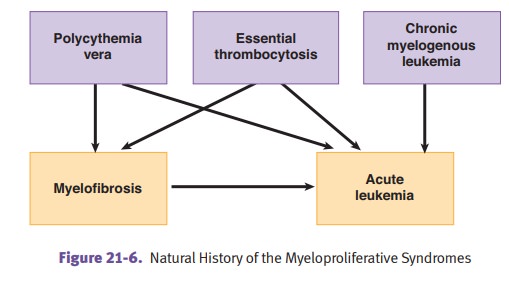
Polycythemia vera is a stem cell disorder with
trilineage (erythroid, granulo-cytic, megakaryocytic) proliferation. It may
develop into a “spent phase” with myelofibrosis. It causes an increased risk
for acute leukemia. Phlebotomy is therapeutic.
Polycythemia vera
characteristically shows the following:
•
Increased erythroid precursors with increased red cell mass
•
Increased hematocrit
•
Increased blood viscosity, which can cause deep vein thrombosis and
infarcts
•
Decreased erythropoietin, but erythrocytes have increased
sensitivity to erythropoietin and overproliferate
•
Increased basophils. Histamine release from basophils can cause intense
pruritus and gastric ulcer (bleeding may cause iron deficiency).
•
Increased eosinophils (like all of the MPS)
•
High cell turnover can cause hyperuricemia, resulting in gout.
Other clinical characteristics include plethora (redness) and cyanosis (blue).
Essential thrombocythemia is characterized by increased
megakaryocytes (and other cell lines) in bone marrow.
Peripheral blood smear shows increased platelets, some with abnormal shapes.
There are also increased leukocytes. Clinical signs include excessive bleeding
and occlusion of small vessels.
Myelofibrosis (MF) with
myeloid metaplasia has unknown etiology (agnogenic).
•
Marrow fibrosis is secondary to factors released from
megakaryocytes, such as platelet-derived growth factor (PDGF).
•
Bone marrow aspiration may be a “dry tap.” The biopsy specimen
shows hypocellular marrow with fibrosis (increased reticulin). The fibroblasts
are a polyclonal proliferation and are not neoplastic.
•
There is an enlarged spleen due to extramedullary hematopoiesis
(myeloid metaplasia). Peripheral smear shows leukoerythroblastosis (immature
white cells and nucleated red cells) with teardrop RBCs. High cell turnover
causes hyperuricemia and gout.
Related Topics