Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Genomics, Other “Omics” Technologies, Personalized Medicine, and Additional Biotechnology Related Techniques
Knockout Mice
Knockout Mice
While many species including mice, zebra fish, nemotodes, etc. have been
transformed to lose genetic function for the study of drug discovery and disease
modeling, mice have proven to be the most useful. Mice are the laboratory
animal species most closely related to humans in which the knockout technique
can be easily performed, so they are a favorite subject for knockout
experiments, While a mouse carrying an introduced transgene is called a
transgenic mouse, transgenic technologies can also produce a knockout animal
(mice are the most studied animal species). A knockout mouse, also called a
gene knockout mouse or a gene-targeted knockout mouse, is an animal in which an
endogenous gene (genomic wild-type allele) has been specifically inactivated by
replacing it with a null allele (Mak et al., 2001; Lesney, 2003; Sharpless and
DePinho, 2006). A null allele is a nonfunctional allele of a gene generated by
either deletion of the entire gene or mutation of the gene resulting in the
synthesis of an inactive protein. Recent advances in intranuclear gene
targeting and embryonic stem cell technologies as described above are expanding
the capabilities to produce knockout mice routinely for studying certain human
genetic diseases or elucidating the function of a specific gene product.
The procedure for producing knockout mice basically involves a four-step
process. A null allele (i.e., knockout allele) is incorporated into one allele
of murine ES cells. Incorporation is generally quite low; approximately one
cell in a million has the required gene replacement. However, the process is
designed to impart neomycin and ganciclovir resistance only to those ES cells in
which homologous gene integration has resulted. This facilitates the selection
and propa-gation of the correctly engineered ES cells. The resulting ES cells
are then injected into early mouse embryos creating chimeric mice (heterozygous
for the knockout allele) containing tissues derived from both host cells and ES
cells. The chimeric mice are mated to confirm that the null allele is
incorporated into the germ line. The confirmed heterozygous chimeric mice are
bred to homogeneity producing progeny that are homozygous knockout mice.
Worldwide, three major mouse knockout programs are proceeding in colla-boration
to create a mutation in each of the approxi-mately 20,000 protein-coding genes
in the mouse genome using a combination of gene trapping and gene targeting in
mouse embryonic stem (ES) cells (Staff, 2007). These include: (1) KOMP
(KnockOut Mouse Project, http://www.knockout-mouse.org), funded by the NIH; (2)
EUCOMM (EUropean COnditional Mouse Mutagenesis examining the role of each gene in normal physiology and development, and shed light on the pathogenesis of abnormal physiology and disease. RNA silencing and interference techniques (siRNA and RNAi) are impacting knockout mice production and are discussed.
Extensive previous research has generated and the continuing discoveries
of the three worldwide mouse knockout consortia will further create better
models of human monogenic and polygenic diseases such as cancer, diabetes,
obesity, cardiovascular disease, and psychiatric and neurodegenerative
dis-eases. For example, knockout mice have been en-gineered that have extremely
elevated cholesterol levels while being maintained on normal chow diets due to
their inability to produce apolipoprotein E (apoprotein E) (Zhang et al., 1992;
Breslow, 1994). Apoprotein E is the major lipoprotein component of very
low-density lipoprotein (VLDL) responsible for liver clearance of VLDL. These
engineered mice are being examined as animal models of atherosclerosis useful in
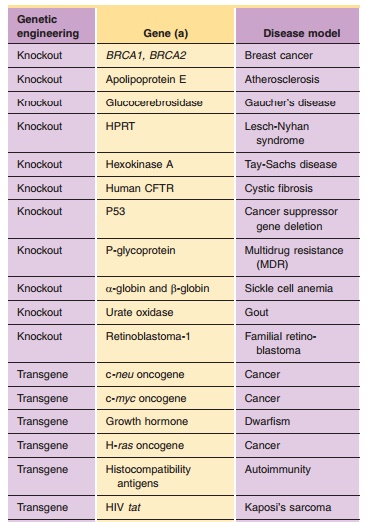
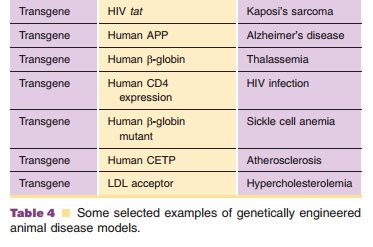
cardiovascular drug discovery and develop-ment. Table 4 provides a list of some
additional selected examples of knockout mouse disease models.
The knockout mouse is becoming the basic tool for researchers to
determine gene function in vivo in numerous biological systems. For example,
knockout mouse technology has helped transform our under-standing of the immune
response (Mak et al., 2001). The study of single and multiple gene knockout
animals have provided new perspectives on T-cell develop-ment, co-stimulation
and activation. “Humanized mice,” transgenic Severe Combined Immunodeficient
(SCID) mice grafted with human cells and tissues enable research in
regenerative medicine, infectious disease, cancer, and human hematopoiesis. In
addition, high-throughput DNA sequencing efforts, positional cloning programs,
and novel embryonic stem cell-based gene discovery research areas all exploit
the knockout mouse as their laboratory.
Engineered animal models are proving invalu-able to pharmaceutical
research since small animal models of disease may be created and validated to
mimic a disease in human patients. Mouse, rat, and Zebrafish are the most
common models explored and used. Genetic engineering can predispose an animal
to a particular disease under scrutiny and the insertion of human genes into
the animal can initiate the development of a more clinically relevant disease
condition. In human clinical studies, assessments of efficacy and safety often
rely on measured effects for surrogate biomarkers and adverse event reporting.
Validated transgenic animal models of human disease allow for parallel study
and possible predictability prior to entering clinical trials. Also, it is
possible to screen potential drug candidates in vivo against a
human receptor target inserted into an animal model. The number of
examples of transgenic animal models of human disease useful in drug discovery
and development efforts is growing rapidly (Sharpless and DePinho, 2006;
Schultz et al., 2007). Such models have potential to increase the efficiency
and decrease the cost of drug discovery and development by reducing the time it
takes to move a medicinal agent from discovery into clinical trials. Table 4
provides a list of some selected examples of genetically engi-neered animal
models of human.
Related Topics