Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Kinetic Methods of Analysis
Kinetic Methods of Analysis
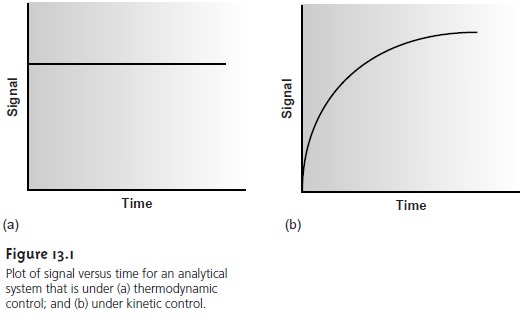
Asystem under
thermodynamic control is in a state of equilibrium,
and its signal has a constant, or steady-state value
(Figure 13.1a). When a
system is under
kinetic control, however,
its signal changes
with time (Figure 13.1b)
until equilibrium is established. Thus
far, the techniques we have considered have involved measurements made when the system is at equilibrium.
By
changing the time at which measurements are made, an analysis
can be carried out under either
thermodynamic control or kinetic
control. For example, one method
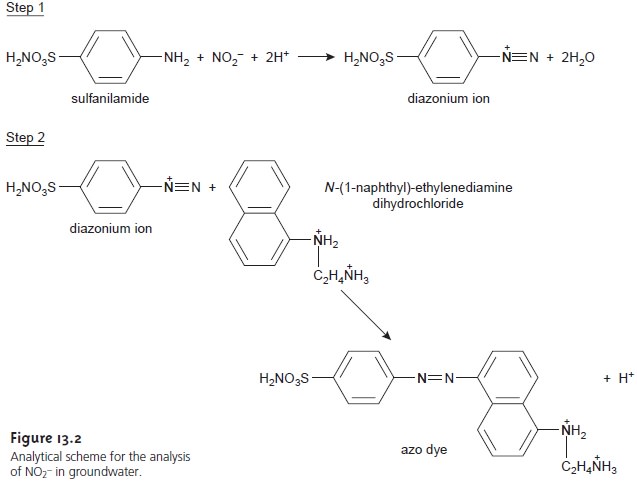
for determining the concentration of NO3–
in groundwater involves the
diazotization reaction shown in Figure
13.2. The final
product, which is a reddish-purple azo
dye, absorbs visible light at a
wavelength of 543 nm. Since the concentration of dye is determined by the amount of NO3–
in the original sample, the
solution’s absorbance can be used to determine
the concentration of NO3–. The
reaction in the second step, however, is not instantaneous. To achieve a steady-state signal, such as that in Figure 13.1a, the
absorbance is measured
following a 10-min delay. By measuring
the signal during
the 10-min development period, information about the
rate of the reaction is obtained.
If the reaction’s rate is a function
of the concentration of NO3–, then the
rate also can
be used to determine its
concentration in the sample.
There are many potential advantages to kinetic methods
of analysis, perhaps the most important of which is the ability
to use chemical reactions that are
slow to reach
equilibrium. In this
we examine three techniques
that rely on measurements made while the analytical system is under kinetic
rather than thermodynamic control: chemical
kinetic techniques, in which the rate of a chemical
reaction is measured; radiochemical techniques, in
which a radioactive element’s rate of nuclear
decay is measured; and flow injection analysis, in which
the analyte is injected
into a continuously flowing carrier
stream, where its mixing and reaction with reagents in the stream
are controlled by the
kinetic processes of convection and
diffusion.
Related Topics