Chapter: Essentials of Psychiatry: Cognitive Enhancers and Treatments for Alzheimer’s Disease
Cognitive Enhancers and Treatments for Alzheimer’s Disease
Cognitive Enhancers and
Treatments for Alzheimer’s Disease
By the
late 1980s the advent and general acceptance of research-based diagnostic
criteria for the dementia of Alzheimer’s disease (AD) (McKhann et al., 1984), an understanding of its
underlying pathology along with mechanism-based pharmacological therapeu-tics
provided the framework for clinical trials to exploit a variety of new
treatment strategies that might positively impact the illness.
Since AD
is defined by the presence of dementia, attempts have been made to identify a
predementia state of cognitive im-pairment, likely to lead to AD. This state of
“mild cognitive im-pairment” (MCI) has now been the target of several clinical
tri-als of medications previously used for AD. However, MCI is not merely a
predementia stage of AD, since many people who fulfill criteria do not progress
to dementia.
Regulatory Issues
The Food
and Drug Administration (FDA) utilizes de
facto guidelines for establishing that a drug has “antidementia effi-cacy”
(Leber, 2002). These require, in part, that: 1) clinical tri-als be double-blind
and placebo-controlled; 2) patients fulfill the now-accepted criteria for a
primary dementia such as AD (e.g., using either DSM-IV-TR or NINCDS-ADRDA
(National Insti-tute of Neurological and Communicative Disorders and
Stroke-Alzheimer’s Disease and Related Disorders Association) Work Group
Criteria) (McKhann et al., 1984); and
3) appropriate effi-cacy instruments be used. Although the de facto guidelines avoid specifying that only Alzheimer’s dementia
can be treated, allow-ing the possibility that any recognized or accepted
conditions can receive approval, at present it is the only dementia for which
FDA-approved medications are available. [Note that DSM-IV-TR criteria for
Dementia of the Alzheimer’s Type very closely reflect the NINCDS-ADRDA Work
Group Criteria.]
Limitations
to the current guidelines include the failure to recognize improvement in
behavior or functional activities alone
as legitimate therapeutic goals or indications in the prescribing information,
despite the fact that behavioral symptoms occur in the majority of dementia
patients, and that improvements in functional status may have a major effect on
prolonging inde-pendence. In addition, these guidelines fail to provide for
effi-cacy measures for severely impaired patients who are unable to perform
standard cognitive tests.
Therapeutic Implications of Pathophysiology
Advances
in understanding of plaques and tangles over the last few years underscore the
biological heterogeneity of the illness, and several clues about definitive
therapeutic approaches have appeared. It is likely that there are several
stimuli for these ab-normal protein processes, genetically, biologically and
environ-mentally determined. These processes need to be understood more fully
in order to discover new drugs acting directly on the pathological processes
responsible for the neurodegeneration. For example, the finding that amyloid
precursor protein process-ing is in part controlled by a cholinergic mechanism
suggests that cholinergically based therapeutic strategies may modify the
progress of the disease as well as providing symptomatic relief (Giacobini,
1996).
Other
potential interventions in development now include agents that interfere with
beta-amyloid formation such as secre-tase inhibitors, modulators of APP
expression, β-secretase
and β-secretase inhibitors that
prevent cleavage of APP into insoluble β -amyloid protein, inhibitors of β-amyloid protein aggregation or
deposition, or the passive or active immunization with antibodies to β-amyloid.
The
observed neurotransmitter perturbations in AD, how-ever, provide more immediate
and accessible targets for thera-peutic interventions. For example, the
observation of deficits in noradrenergic or serotoninergic function provide
rationales for the use of antidepressants in patients with AD and symptomatic
behaviors. Although not the exclusive pathological change, the cholinergic
deficits represent the most consistent transmitter de-pletion and appear to be
one of the early events in the disease process (Francis et al., 1985, 1999). Thus, it continues to remain a major focus of
applied clinical pharmacological research.
Treatment Paradigms
Approaches
to the treatment of AD can be grouped into several conceptual categories (Table
83.1). One approach attempts to treat the behavioral symptoms such as
agitation, aggression, psy-chosis, depression, anxiety, apathy, and sleep or
appetite distur-bances. A second approach attempts to treat the cognitive or
neu-ropsychological signs symptoms of the illness such as memory, language,
praxis, attention, orientation and knowledge. A third
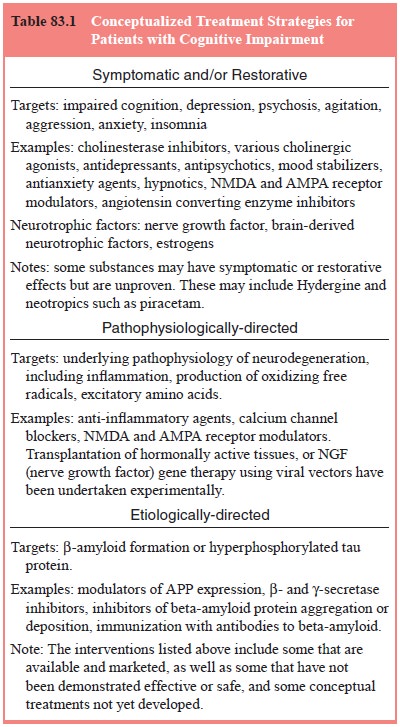
approach
attempts to slow the rate of progression of the illness, preserving patients’
quality of life or autonomy. (Slowing the rate of decline might also be related
to treating symptoms.) A fourth conceptual treatment approach is primary
prevention, to delay the time to onset of illness. Success at this approach
could have considerable impact.
Cholinergic Agents
The
primary implication of the cholinergic hypothesis is that potentiation of
central cholinergic function should improve the cognitive and behavioral
impairment associated with AD. This simple “neurotransmitter replacement”
rationale has been made most compelling by the consistent effects of
cholinesterase in-hibitors as a class of drugs across trials.
While
agents with several kinds of procholinergic action have been evaluated for
efficacy in AD, the ChIs (cholinesterase inhibitors) are the only agents to
have consistently demonstrated efficacy in numerous multicenter,
placebo-controlled trials, and thus have been approved by many national
regulatory authorities Thus ChIs represent the first class of efficacious pharmacological approaches for AD, and an
approach that is likely to be clinically useful for the indefinite future,
especially since research on drugs with other mechanisms has not advanced as
rapidly as had been hoped for.
The well-established
cholinergic defects in AD include: decline of cholinergic baso-cortical
projections; reduced activity of ChAT, the key acetylcholine (ACh) synthesis
en-zyme, and cholinergic cell body loss in the nucleus basalis. Additionally,
there are correlations between cortical ChAT reduction or nucleus basalis cell
reduction and cortical plaque density. Such cholinergic deficits correlate with
cognitive de-cline as measured by the Blessed-Roth Dementia Rating Scale
(Blessed et al., 1968). The cholinergic
hypothesis proposes that cognitive deficits of AD are related to decreases in
central acetylcholinergic activity, and that increasing intrasynaptic ACh will
enhance cognitive function and clinical well-being. (See Figure 83.1.)
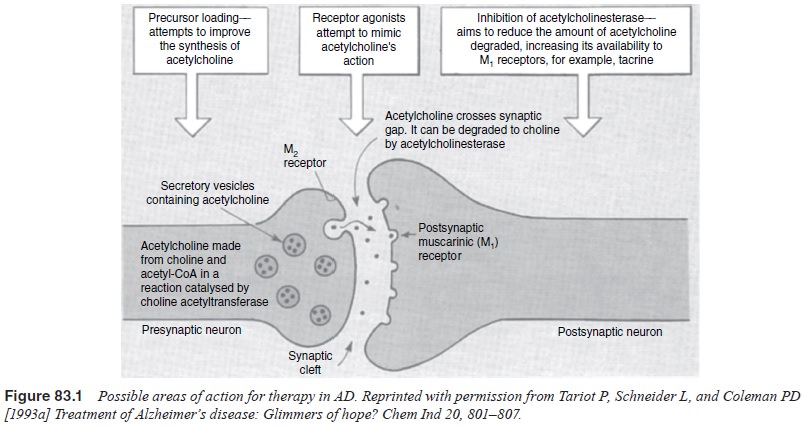
Cholinergic Treatment Approaches
Cholinergic
treatment approaches include precursor loading, cholinesterase inhibition,
direct cholinergic receptor stimulation and indirect cholinergic stimulation.
Unfortunately, most of these cholinergic strategies have thus far proven ineffective,
effective but too toxic, or have not been completely developed.
Cholinesterase Inhibitors
ChIs have
shown generally consistent symptomatic efficacy in standardized,
well-controlled multicenter trials lasting from 6 months to occasionally 12 months.
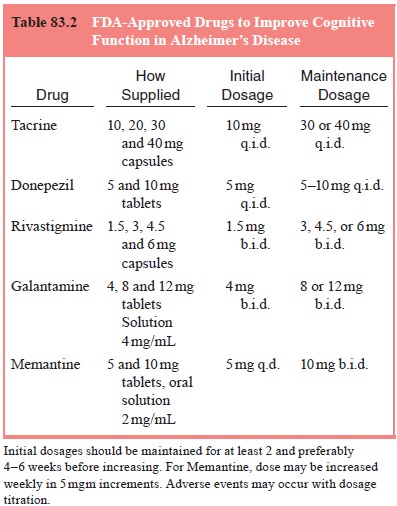
Cholinesterase inhibitors (Table 83.2) have been the most frequently used
experimental treatment for AD and the major group of medications to yield
consistently positive results in clinical trials. Current marketed ChIs include
tacrine, donepezil, rivastigmine and galantamine, although tacrine is now used
much less commonly due to the risk of hepatotoxicity.
Mechanisms of Cholinesterase Inhibition
Acetylcholine
is inactivated when it is hydrolyzed to choline and acetate by
acetylcholinesterase (AChE). By inhibiting the actions of AChE, ChIs
effectively increase the amount of ACh available for intrasynaptic cholinergic
receptor stimula-tion. A summary of pharmacokinetics and pharmacodynamics is in
Table 83.3.
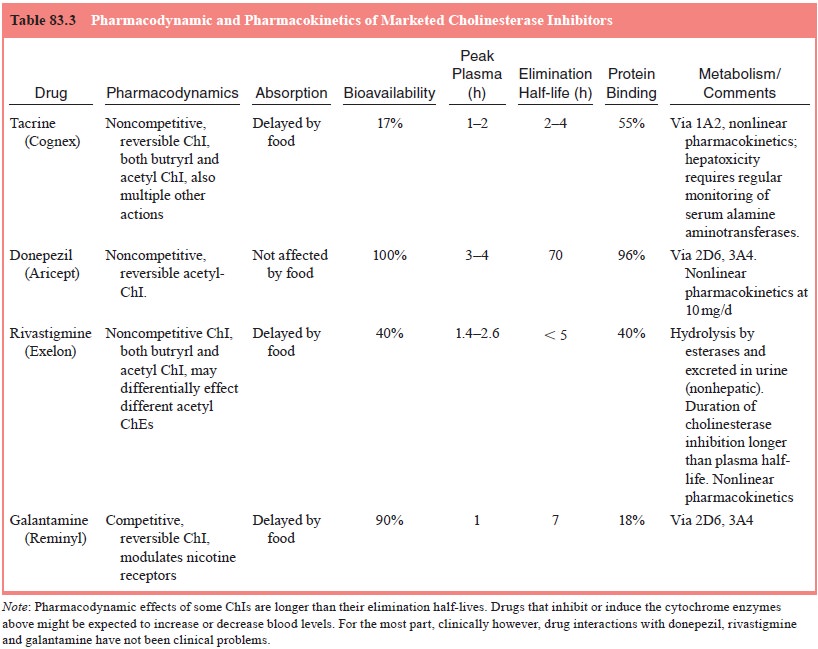
Individual Cholinesterase Inhibitors – Dosing and Adverse Effects
Tacrine
Tacrine
is a noncompetitive reversible inhibitor of ChE. It binds near the
catalytically active site of the AChE molecule to inhibit enzyme activity. It
has other actions as well including blocking sodium and potassium channels, and
direct activity at muscarinic receptors (Adem et al., 1990).
Dosing
Tacrine’s FDA-approved dosing regimen is based on the clini-cal trials. The recommended starting dose is 10 mg q.i.d. to be maintained for 6 weeks, while serum transaminase levels are monitored every other week. If the drug is tolerated and transam-inase levels do not increase above three times the upper limit of normal, the dose is then increased to 20 mg q.i.d. After 6 weeks, dosage should be increased to 30 mg q.i.d., again with biweekly monitoring and then, if tolerated, to 40 mg q.i.d. for the next 6 weeks. Due to hepatotoxicity concerns, Tacrine’s use greatly decreased.
Donepezil
Donepezil
(Aricept™) is a long-acting piperidine-based highly selective and reversible
acetylcholinesterase inhibitor.
Dosing
Donepezil
is initiated at 5 mg/day and then increased to 10 mg/day after 4 to 6 weeks.
Raising the dose earlier increases the risk for cholinergic adverse events.
Five or 10 mg/day are effective doses; 10 mg tends to be somewhat more
effective than 5 mg when the various trials as a group are evaluated.
Rivastigmine
Rivastigmine
(Exelon™) is a pseudo-irreversible, selective AChE subtype inhibitor.
Dosing
The
recommended starting dose of rivastigmine is 1.5 mg b.i.d., taken with meals.
If this dose is well tolerated after a minimum of 2 weeks of treatment, it may
be increased to 3 mg b.i.d. Sub-sequent increases to 4.5 mg and then 6 mg
b.i.d. should be based on good tolerability of the current dose and may be
considered after a minimum of 2 weeks of treatment. Higher daily doses,
averaging about 9 to 10 mg were associated with better efficacy than lower
doses.
Galantamine
Galantamine (Reminyl™), an alkaloid originally extracted from Amaryllidaceae (Galanthus woronowi, the Caucasian snow-drop), but now synthesized, is a reversible, competitive inhibi-tor of AChE with relatively less butyrylcholinesterase activity (Harvey, 1995).
Dosing
Initial
dosing is 4 mg b.i.d., and should be raised to 8 mg b.i.d. after 2 to 4 weeks.
For patients who are tolerating medication but not responding the dose can be
raised to 12 mg b.i.d. after another 4 weeks.
Adverse Effects of Cholinesterase Inhibitors
Most
adverse events from ChIs are cholinergically mediated, and are
characteristically mild in severity and short-lived, lasting only a few days.
Adverse events of the marketed ChIs are sum-marized in Table 83.4. Significant
cholinergic side effects can oc-cur in up to about 25% of patients receiving
higher doses. Often they are related to the initial titration of medication.
Patients tend rapidly to become tolerant to the adverse events when they
oc-cur. Hepatotoxicity with tacrine is a significant concern requiring very
close monitoring if the drug is used.
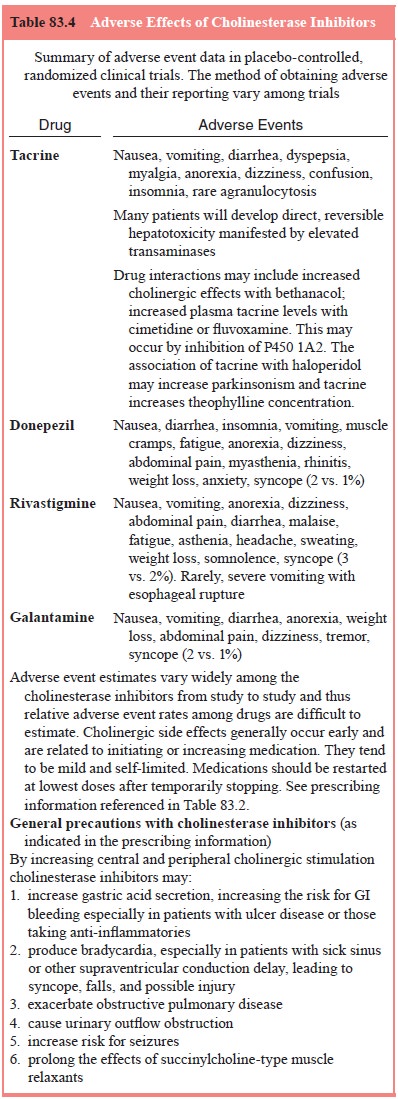
Because
of the actions of ChIs, these drugs require caution when used in patients with
significant asthma, significant chronic obstructive pulmonary disease, cardiac
conduction defects, or clinically significant bradycardia. Appropriate
considerations are involved in general anesthesia as well since they may
prolong the effects of succinylcholine-type drugs.
Drug
interactions with medications which inhibit cy-tochrome P450 types 3A4 or 2D6
may occur with this class of medications.
Infrequent Adverse Events Worth Noting
A number
of infrequent adverse events that may be of particular concern to patients,
caregivers and physicians, and are common among the class of ChIs include
fatigue, anorexia, weight loss and bradycardia. Myasthenia and respiratory
depression occurred in a few patients treated with the higher doses of the
organophos-phate drug metrifonate leading to its therapeutic demise for AD.
Although myasthenia might not be expected to occur with the reversible ChI, physicians
should be vigilant for complaints of fatigue and weakness.
An
increased but modest incidence of anorexia appears to be a consistent finding
across clinical trials and appears to be dose related. The absolute reported
incidence var-ies across trials from approximately 8 to 25% at the highest dose
of ChI compared with 3 to 10% in comparable placebo patients. Similarly, there
is an increased rate of significant weight loss with higher doses of ChIs
compared with placebo patients. The proportion of patients losing greater than
7% of their baseline weight varies from approximately 10 to 24% in the higher
doses and from 2 to 10% of the placebo-treated patients in those trials
reporting the statistic. Anorexia and weight loss are significant clinical
problems for many elderly patients independent of medication effects, and
whether or not demented.
Treatment Approach with Cholinesterese Inhibitors
The
typical candidates for ChIs are outpatients with AD of mild to moderate
cognitive severity. They usually live at home or in an assisted living
facility. Dementia is their main clini-cal problem; concurrent illnesses are
not severe or dominating the clinical picture. Nor do behavioral syndromes such
as psy-chosis, agitation, or significant insomnia, apathy, or depression
dominate.
As
indicated above, dosing should be initiated with 5 mg/ day donepezil, 1.5 mg
b.i.d. rivastigmine or 4 mg b.i.d. galan-tamine. Tacrine should be reserved as
a second-line medication since it requires q.i.d. dosing and biweekly blood
monitoring for elevated transaminase. After a minimum of 2 weeks, but
prefer-ably 4 to 6 weeks, the dosages should be doubled although 5 mg of
donepezil is an effective dose.
Optimal
duration of treatment with continuing efficacy is unknown but overall efficacy
extends at least 9 to 12 months based on the clinical trials and open-label
extension phases.
Maintenance
treatment can be continued as long as a therapeutic benefit for the patient
seems apparent. Therefore, the potential clinical benefit of ChIs should be
reassessed on a regu-lar basis. Discontinuation should be considered when
evidence of a therapeutic effect is no longer present. Because of the great
interpatient variability of response, it is not possible to predict individual
patient responses to ChIs.
It is
difficult to assess individual patient response because of the variability of
the deteriorating course of AD, and because most of the effect of medication is
due to a stabilization or lack of worsening of symptoms or cognitive function
while placebo-treated patients continue to decline. Therefore, the clinical
ob-servations of minimal or no clinical worsening may be sufficient reasons to
continue medication treatment if patients are tolerat-ing therapy.
Monitoring Side Effects
Cholinergic
side effects such as diarrhea, nausea and vomiting, when they occur, tend to
occur at initiation of treatment and when titrating to higher doses. They are
often transient or self-limited and can often be managed with encouragement and
maintenance of the present dose level, by omitting one or more doses, or by
temporarily decreasing dosage. Most cholinergic side effects are related to the
dose escalation phase of treatment, just after start-ing or increasing.
Patients on maintenance doses should have few and very mild cholinergic side
effects if any.
However,
anorexia and weight loss may be clinically sig-nificant problems over the
longer term, especially in older, more medically ill and nursing home patients,
so these parameters should be monitored and medication reduced or discontinued
if anorexia or weight loss become clinically significant to assess if appetite
returns.
Uncommonly,
the vagotonic effects of ChIs may cause significant bradycardia, and this can
be a particular concern to patients with supraventricular conduction
impairments or sick sinus syndrome.
Because
gastric acid secretion may be increased with cholinesterase inhibitors, there
may be an increased risk for de-veloping ulcers or gastrointestinal bleeding.
Patients receiving nonsteroidal anti-inflammatory drugs may be at a
particularly additive risk. It is possible that ChIs may cause bladder out-flow
obstruction, seizures and exacerbate asthma or obstruc-tive pulmonary disease,
and interfere with succinylcholinelike anesthetics.
Effect on Behavior
The
evidence that ChIs may improve behavior is based on case series and secondary
analyses of efficacy trials (Kaufer et al.,
1996; Raskind et al., 1997).
Nevertheless, clinical experience suggests that they may be effective at least
for mildly disturbed behavior, and in delaying the onset of troublesome
behaviors, perhaps by maintaining cognitive function or perhaps through
enhancing attentional processes and activation.
Neuroprotection
Cholinergic
therapies may have effects beyond the short-term symptomatic improvement of
cognition and may modify the pathogenetic processes of the illness (Radebaugh et al., 1996; Thal et al., 1997). For example, activation of M1 muscarinic re-ceptors can stimulate secretion of
amyloid precursor proteins via the α-secretase
pathway such that there is a decrease in the production of toxic and insoluble β-amyloid, thus theoretically
decreasing the formation of amyloid plaques and promoting the normal processing
of APP (Inestrosa et al., 1996;
Muller et al., 1997; Nitsch et al., 1992). These effects remain to
be proven in clinical trials (Buxbaum et
al., 1992; Haroutunian et al.,
1997; Lahiri et al., 2000).
Memantine
L-glutamate
is the main excitatory neurotransmitter in the central nervous system.
Enhancement of its activity at the N-methyl-D-aspartate (NMDA) receptor may
contribute to the pathogenesis of Alzheimer’s disease, a phenomenon known as
excitotoxicity. Memantine is a low affinity antagonist that is believed to
reduce NMDA receptor overstimulation and restore receptor signalling function
to more normal levels. It is also possible that reducing excitotoxicity may be
neuroprotective by preventing neuronal cal-cium overload though there is no
evidence that memantine modi-fies neurodegeneration in patients with
Alzheimer’s disease.
Unlike
the cholinesterase inhibitors, memantine is ap-proved by the FDA for the
treatment of moderate to severe Alzhe-imer’s disease (defined as an MMSE score
of less than 15).
Dosing
Treatment
should be initiated at a dose of 5 mg/day and increased in increments of 5 mg
at intervals of one week to a target dose of 20 mg/day; doses of 10 mg/day or
more should be divided into two given 12 hours apart. Memantine is well
tolerated and is not associated with an increase in treatment discontinuation
com-pared with placebo.
Related Topics