Changes Around Us | Term 2 Unit 3 | 6th Science - Classification of Changes | 6th Science : Term 2 Unit 3 : Changes Around Us
Chapter: 6th Science : Term 2 Unit 3 : Changes Around Us
Classification of Changes
Classification
of Changes
There
are different types of changes observed in nature that occurs around us. Some
changes take place very quickly while others take hours, days or even years.
Some changes are temporary while some others are permanent. Some changes
produce new substances while others do not. Some changes are natural while
others are made by human beings. Some changes are desirable to us but some
changes are not desirable.
We
shall now try to classify changes on the basis of certain similarities and
differences.
* slow and fast
* reversible and
irreversible
* physical and
chemical changes
* desirable and
undesirable
* natural and man – made
1. Slow and Fast
changes
Activity 2: Look at the pictures and discuss
about the duration for the changes to take place

Slow changes
Changes which take place over a long period of
time ( hours / days / months / years ) are known as Slow changes.
Examples: growth of nail and hair, change of seasons, germination of seed.
Fast Changes
Changes which take place within a short period
of time (seconds or minutes) are known as fast changes.
Examples: Bursting of balloon, breaking of glass, bursting of fire
crackers, burning of paper.
2.
Reversible and Irreversible changes
Reversible change
Changes which can be reversed (to get back the
original state) are known as reversible changes.
Examples: Touch me not plant (Responding to touch), stretching of rubber
band, melting of ice.

Touch me Plant
Activity
3: Try to make a boat and an aeroplane
one by one using the same piece of paper. This means the change of shape
discussed here is reversible.
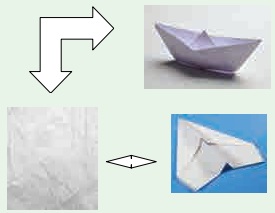
Irreversible change
Changes which
cannot be reversed or to get back the original state are known as Irreversible
changes.
Activity 4:
What kind of changes are they?

a) Burning of a candle.
Irreversible change
b) Piercing a balloon with
a pin.
Irreversible change
Examples : Change of milk into curd, digestion of
food, making idly from batter.

3. Physical
and Chemical Changes
Activity 5: Take an apple and cut it into
two halves. Cut one half into pieces and share it with your friends. Is there any change
in the composition of the Apple while cutting?

No, only the shape and size have
changed. This can be called a physical change.
Leave
the other half on the table for some time. You can see brown patches formed on
the cut surface because of the reaction between some substances in the apple
and the air around it. This is a Chemical change.
Physical
changes
Physical changes are the temporary changes in
which there is change in the physical appearance of the substance but not in
its chemical composition. Here no new substance is formed.
Example: Melting of ice, the solution of salt or
sugar, stretching of rubber band.
Let us now
understand the physical changes that take place in water. You already
know that water exists in three states as solid, liquid and gas. Change of
state takes place either by heating or cooling. By heating energy is supplied
and by cooling energy is taken away. These are the reasons for the changes.

Let us name a few
processes connected with the changes in states of water.

More to Know
The change of state from solid to
gas directly is called Sublimation.
Example
: Camphor
Let us understand one more physical change
Dissolution
The spreading of
the solid particles (broken into individual molecules) among the liquid
molecules is called as dissolution.
* Solvent is a substance that dissolves the
solute.
* Solute is a substance that is dissolved in a
solvent to make a solution.
* When solute is
dissolved in a solvent it forms a solution.
Solute + Solvent → Solution
Water is known as the universal
solvent. It dissolves
a wide range of substance.
Activity
6: Take half a cup of water, add
one spoon full of sugar and stir well.

a. What
do you observe?
b. What
happened to the sugar?
c. Where
is it gone?
d. The
solute in the above solution is
e. The solvent in the above
solution is
f. Have you seen a glass of water
and a glass of sugar solution looking alike?
Chemical
changes
Chemical changes are the permanent changes in
which there is change in the chemical composition and new substance is formed.
Examples: Burning of wood, Popping of popcorn,
Blackening of silver ornaments, and Rusting of iron.

Activity
7: Look at the pictures and write
whether they are Physical or Chemical changes.

4. Desirable and Undesirable Changes
Activity
8: Look at the pictures and write
whether they are desirable or undesirable changes.

Desirable
changes
The changes which
are useful, not harmful to our environment and desired by us are known as
desirable changes.
Examples: Ripening of fruit, growth of plants, cooking of food, milk
changing to curd.
Undesirable changes
The changes which are harmful to our
environment and not desired by us are known as Undesirable changes.
Examples: Deforestation, decaying of fruit,
rusting of iron.
5. Natural and human made changes
Activity
9: Identify the type of changes
Natural
/ Human made

Natural changes
Changes which take
place in nature on their own and are beyond the control of human beings are
known as Natural changes.
Examples: Rotation of the earth, Changing phases of the Moon, Rain.
Human made or artificial changes
The changes which are brought about by human
beings are known as human made or artificial changes. They will not happen on
their own.
Examples: Cooking, Deforestation, Cultivating crops, construction of
buildings.
Related Topics