Chapter: Essentials of Psychiatry: Mood Disorders: Bipolar (Manic–Depressive) Disorders
Bipolar (Manic–Depressive) Disorders: Somatotherapy
Somatotherapy
Efficacious Agents for Various Phases of Manic–Depressive Disorder
Over the past 10 years medications have proliferated for the treat-ment
of various phases of the disorder. The clinician must pick through an array of
scientific data and marketing claims in order to choose the appropriate
treatment. Two conceptual approaches help in this task. First, available
scientific data can be reviewed and evaluated according to the techniques of
“evidence-based medicine”.
The second conceptual approach that we have found useful is to propose
an explicit definition for the term “mood stabilizer” and evaluate the role of
various medications against this defini-tion. The US Food and Drug
Administration (FDA) does not for-mally define the term, but it stands to
reason that an agent would be optimally useful for treatment of
manic–depressive disorder if it had efficacy in four roles: 1) treatment of
acute manic symp-toms; 2) treatment of acute depressive symptoms; 3)
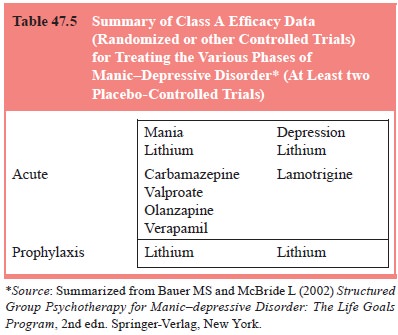
prophylaxis of manic symptoms; and 4) prophylaxis of depressive symptoms Table
47.5 summarizes the findings of this approach.
There are additional Class A controlled trials (nonpla-cebo-controlled)
that support efficacy for multiple older, typi-cal neuroleptics as well as the
benzodiazepines, lorazepam and clonazepam.
In contrast to evidence regarding acute mania, evidence is scarce
concerning efficacy of specific agents for acute
depressive episodes. Most treatment is undertaken primarily by extension
from treatment experience in unipolar depression. In reviewing studies of
agents for the prophylaxis of manic or depressive symptoms, most studies report
recurrence rates without distinguishing between manic and depressive symptoms.
For instance, some studies reported such statistics as time-to-first-episode
without specifying whether the first episode was manic or depressed. Other
studies reported summary statistics for affective symptoms without separating
manic or depressive symptoms. Far and away, the most placebo-controlled support
for any prophylactic agent comes from studies of lithium including studies of
relapse prevention for depression, with support from controlled trials that are
not placebo-controlled for carbamazepine and lamotrigine. The one prophylaxis
study of valproate (Bowden et al.,
2000) showed no difference from placebo (lithium was also found to be no different from placebo in this study, although the
study was under-powered to make definitive conclusions about this comparison).
It may be surprising that, given the paucity of data on treat-ment of
acute depression and prophylaxis of manic–depressive disorder, frequently many
other medications are used chroni-cally in this illness, sometimes as
first-time agents, for instance, valproate or carbamazepine. Although
neuroleptics have acute anti-manic evidence, despite the fact that there is
little evidence for prophylactic efficacy, they are often used chronically. This
is because these agents are typically started during the course of an acute
manic episode and clinicians are reluctant to stop them and switch to a
different agent such as lithium. In addition, many individuals have failed or
have been intolerant of treatment with lithium and they are therefore treated
using the “next best thing”. This is not necessarily suboptimal treatment.
However, it is important that the clinician recognize that data on long-term
prophylactic efficacy is quite scanty for these agents, as it is for many other
agents used in psychiatric practice.
Several additional issues in prophylaxis of manic–depres-sive disorder
deserve comment. First, when is lifetime, or at least long-term, prophylaxis
warranted? After one manic episode? One hypomanic episode? One depressive
episode with a strong fam-ily history of manic–depressive disorder? There is
insufficient empirical evidence with which to make strong recommendations. In
clinical practice without clear guidelines, such decisions need to take into
account the capability of the patient and family inreporting symptoms, rapidity
of onset of episodes, episode se-verity and associated morbidity. Clearly, the
risks of a wait-and-see strategy would be different in a person who had a
psychotic manic episode than in a person who had mild hypomania.
Secondly, can lithium ever be discontinued? Again, there are no solid
data on which to base this decision. However, if lith-ium discontinuation is
contemplated, there is evidence that rapid discontinuation (in less than 2
weeks) is more likely to result in relapse than slow taper (2–4 weeks), with
relapse rates higher in type I than in type II patients. In type I patients,
relapse rates for rapid discontinuation versus slow taper were, respectively,
96 and 73%, whereas in type II patients they were 91 and 33% (Faedda et al., 1993).
Thirdly, a set sequence of treatment for refractory manic– depressive
disorder has yet to be established. In particular, persons with rapid cycling
represent a treatment dilemma. Although anti-depressants may induce rapid
cycling, they often leave the person in a protracted, severe depression.
Switching from one antimanic agent to another often results in resumption of
cycling. Complex treatment strategies may be required, such as anticonvulsants
plus lithium, combinations of anticonvulsants, or adjuvant treat-ment with high
doses of the thyroid hormone thyroxine.
A Simple Treatment Algorithm for Manic–Depressive Disorder
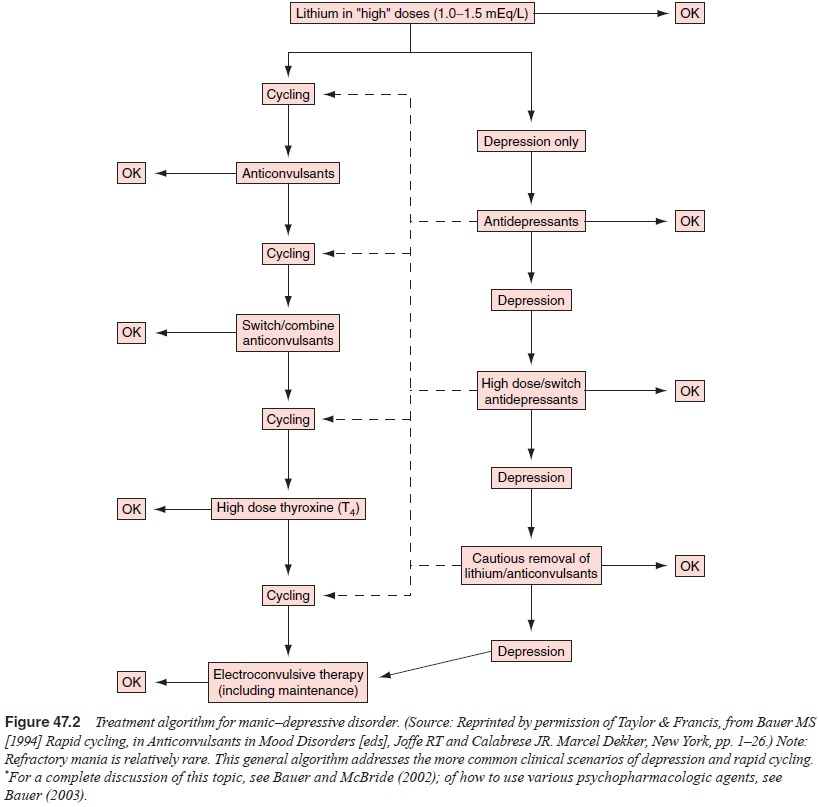
A treatment algorithm for refractory manic–depressive disor-der,
including strategies to deal with rapid cycling is found in Figure 47.2. It is
derived from clinical practice guidelines from the US Veterans Administration
and, by design, primarily speci-fies drug classes rather than individual
agents. The entry point for this algorithm is the occurrence of any major mood
episode (depression, hypomania, mania, or mixed episode) in an unmedi-cated
patient. Patients with recurrence on medications may enter the algorithm at the
appropriate point along the flow diagram. For simplicity of presentation, only
depressive and cycling outcomes are illustrated. This is because depressive
episodes are more common than manic or hypomanic episodes, and all but the most
refractory of the latter episodes are relatively easily treated by the addition
(or resumption) of lithium or anticonvulsants or the use of neuroleptics, as
summarized above.
Balancing Beneficial and Unwanted Effects of Medications
All psychotropic medications have side effects. Some are actu-ally
desirable (e.g., sedation with some antidepressants in per-sons with prominent
insomnia), and specific medications are often chosen on the basis of desired
side effects. However, side effects usually represent factors that decrease a
patient’s quality of life and compromise compliance. Reviews of side effects of
antidepressants and neuroleptics can be found on depression and schizophrenia,
respectively. It should be recalled in regard to antidepressants, however, that
all can cause rapid cycling and mixed states in persons with manic–depressive
dis-order. These effects are not uncommonly encountered in clinical practice
and should be watched for, even in persons taking mood-stabilizing agents.
A brief overview of the most frequent or important side effects of
lithium, carbamazepine and valproic acid can be found in Table 47.6a–c. Note
that some side effects may be encountered at any serum level of the drug, even
within the therapeutic range. Some side effects may be dose-related even within
that range and
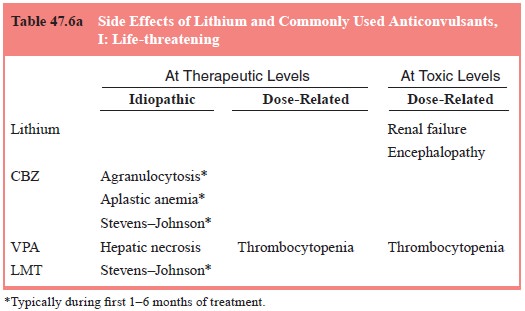
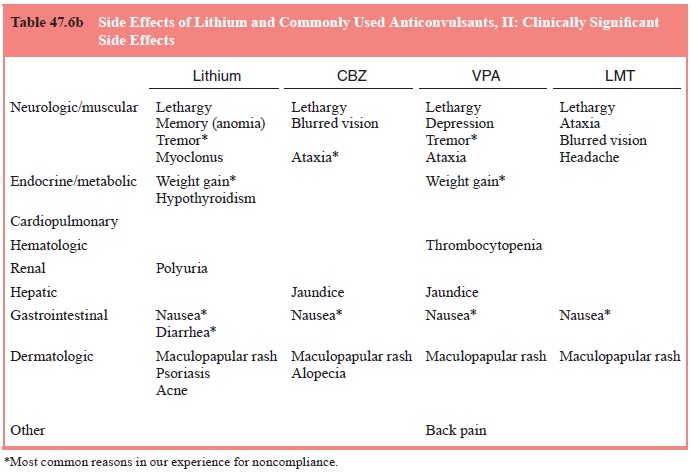
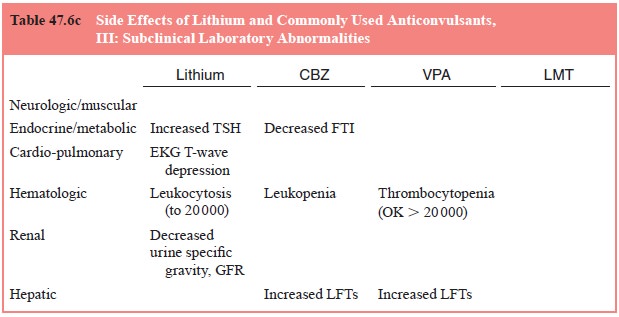
may respond to dosage reduction. Others are more idiosyncratic and may
need other management, as detailed in a subsequent sec-tion. Note that not all
laboratory findings represent pathological processes that are associated with
or presage morbidity for the patient; that is, not all are clinically
significant.
Note also that the concept of the “therapeutic level” is not
straightforward. The lower limit is usually established by the lowest level
necessary for therapeutic effect, whereas the up-per limit is set by the lowest
level associated with regular, sig-nificant toxicity. This range is never
established with complete precision. For some medications such as lithium, the therapeutic
window is actually quite narrow, with toxic effects developing with some
regularity after the upper limit of the therapeutic range is surpassed and with
serious toxicity developing at only mod-estly higher serum levels. As a further
complication, for many persons the minimum level of lithium for good response
may be substantially above the 0.5 to 0.8 mEq/L that is usually set as the
lower therapeutic limit, but this is reached only at the cost of increased
incidence of side effects. On the other hand, experi-ence with valproic acid,
the upper limit of the therapeutic range for mood stabilization may actually be
125 mg/dL rather than the listed range of 100 mg/dL usually accepted for
antiepileptic effect, and this level may be reached without undue side effects.
Thus, established therapeutic levels should be used as important guidelines,
and therapeutic levels should only be exceeded with careful monitoring.
However, one must not be falsely reassured that reaching the lower level of a
therapeutic range is equally ef-fective for all patients, while taking with a
grain of salt the upper limits of the therapeutic range in drugs with a wider
therapeutic window.
Another important issue to consider is drug–drug interac-tions, which
may lead to side effects. Such interactions are often associated with increases
in serum levels of the drug of interest. For example, addition of thiazide
diuretics, or nonsteroidal an-tiinflammatory agents, the latter available over
the counter, is a common reason for increase in lithium level and development
of toxicity. However, at other times the drug–drug interactions may not be
reflected in an increased serum level if the main interac-tion is displacement
of protein-bound drug. Because free drug concentrations are usually 1 to 10% of
total serum drug, a dis-placement of even 50% of bound drug may be associated
with negligible if any changes in total serum level. However, since both
therapeutic and toxic effects are due to free, not bound, drug, unwanted side
effects may develop despite total drug levels measured in the therapeutic
range.
Guiding Principles of Managing Side Effects
Although some side effects may be desirable, in many cases they are
impediments to treatment, frequently of sufficient importance to lead to noncompliance.
Clinicians might reframe the noncom-pliance issue more appropriately as
“insufficient provider–patient cost–benefit analysis”. Stressing compliance
when a person suf-fers from significant side effects is usually much less
effective than working to set appropriate expectations of the patient and to
find a regimen of minimal toxicity. Managing side effects is as much
psychotherapeutic as medical.
There are several strategies available to improve patients’ tolerance of
medications. First, dose reduction may be achieved without compromising
efficacy in some patients. Some side ef-fects, such as lithium-induced nausea,
usually respond well to this, whereas others, such as lithium-induced memory
loss, im-prove less reliably. Secondly, simple changes in preparation may be
helpful, such as using enteric-coated lithium. Uncoated valp-roic acid causes
nausea so frequently that only the coated forms are routinely used; however,
the pediatric “sprinkle” preparation may be of some benefit in persons with nausea
even with enteric-coated valproic acid. Thirdly, changing the administration
sched-ule may ameliorate side effects. Commonsense strategies such as taking
nausea-inducing medications after a meal should not be overlooked. Single daily
dosing of lithium, carbamazepine, or valproic acid may decrease daytime
sedation without compro-mising efficacy. For more obscure reasons, single daily
dosing of lithium appears to decrease polyuria quite effectively.
Fourthly, addition of medications to counteract side effects can
sometimes be the only way to continue treatment. Addition of beta-blockers can
reduce lithium- or valproic acid-induced tremors. Judicious use of thiazide
diuretics, often in conjunction with potassium-sparing diuretics or potassium
supplements, can reduce lithium-induced polyuria. Finally, change to another
drug may be the only alternative. This is clearly indicated in the case of
serious allergic reactions. Polypharmacy should be avoided wherever possible.
Psychotherapies
It is important to note that psychotherapy has been studied almost
exclusively in the context of ongoing medication management, rather than as a
substitute for, or alternative to, medication treat-ment. Rather, psychotherapy
has been utilized as an adjuvanttreatment to optimize outcome in the illness.
Psychotherapy has been viewed as having one or more of several roles in the
man-agement of the disorder.
Recall that both somatic therapies and psychotherapies to date have been
predominantly oriented toward improving clinical outcome. Under this
conceptualization psychotherapy has been thought directly to address symptoms,
such as cognitive therapy for depressive symptoms. Less frequently has
psychotherapy been developed with an explicit component geared toward
addressing the functional deficits in manic–depressive disorder. However,
functional outcome has often been measured in formal trials of various types of
psychotherapy. A third conceptualization has been to use psychotherapy as a
predominantly educative method to assist patients in participating more
effectively in treatment. In this latter regard, treatment is geared toward
improving “host factors”, that is, those factors not directly due to the
disease but that have an impact on its course or treatment, through education,
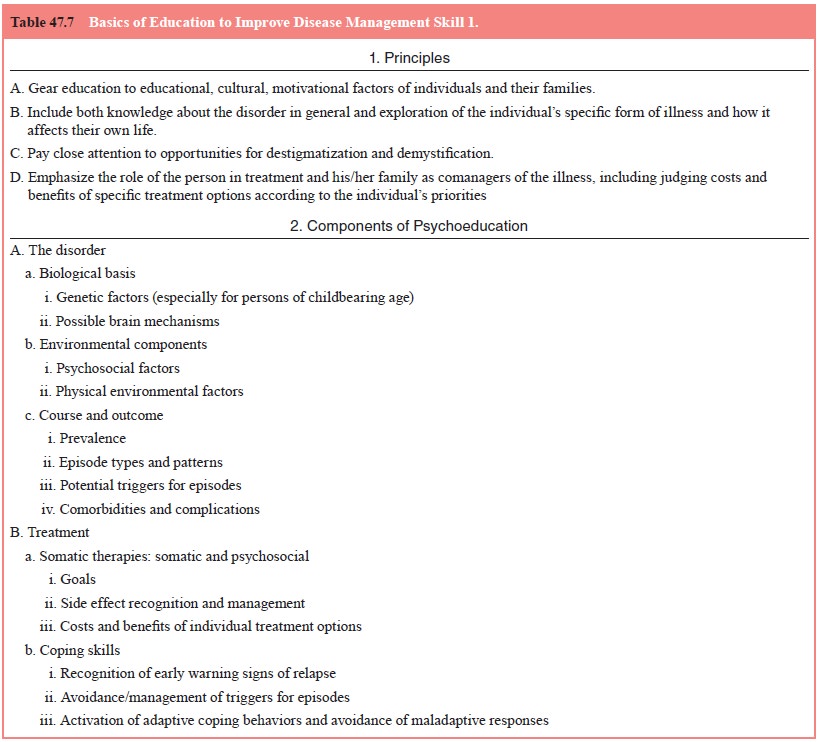
support and problem solving. Such host factors include illness management
skills, which may be improved through psychoedu-cation and attention to
building the therapeutic alliance. Basics of education are summarized in Table
47.7.
An evidence-based review similar to that for somatother-apy has recently
been carried out for psychotherapeutic inter-ventions. Bauer and McBride (2002)
identified five main types of psychotherapy that have been studied in
manic–depressive disorder: couples–partners, group interpersonal or
psychoeduca-tive, cognitive–behavioral, family, and interpersonal and
social-rhythms. Couples–partners, cognitive–behavioral and family methods all
have some Class A data supporting a role in improv-ing clinical outcome or
functional outcome or the intermediate outcome variable of improving illness
management skills. An ad-ditional finding in this review is the degree of
convergent validity across interventions regarding agenda for disease
management information and skills to be imparted. Specifically, imparting
education, focusing on early warning symptoms and triggers of episodes, and
developing detailed and patient-specific action plans are found across most of
the other interventions as well. For instance, this core agenda is also an important
part of such diverse approaches as the cognitive–behavioral interventions of
Palmer and Williams (1995) and Lam and coworkers (2000); the psychoeducational
interventions of Bauer and coworkers (1998), Perry and coworkers (1999) and
Weiss and coworkers (2000); the interpersonal and social rhythms therapy
(IPSRT) intervention of Frank and coworkers (1999); and the family intervention
of Miklowitz and coworkers (1999). Thus, given the positive results most of
these interventions with explicit disease management components (i.e., patient
education, collaborative management strategies with the patient, inclusion of
as wide a social support system as is available) have produced, it is likely
that this basic approach will be critical. It will perhaps be more critical
even than the specific type of intervention in which these disease man-agement
components are embedded
Related Topics