Atomic Structure | Chemistry | Science - Answer the following questions | 9th Science : Chemistry : Atomic Structure
Chapter: 9th Science : Chemistry : Atomic Structure
Answer the following questions
CHEMISTRY
ATOMIC
STRUCTURE
TEXT BOOK EXERCISES
VI. Answer very
briefly:
1. Name an element which has the
same number of electrons in its first and second shell.
Answer: Beryllium.
Atomic number - 4 (K-shell-2, L-shell-2)
2. Write the electronic
configuration of K and CI
Answer:

3. Write down the names of the
particles represented by the following symbols and explain the meaning of
superscript and subscript numbers attached. 1H1, 0n1,
-1e0
Answer:
1H1 : hydrogen atom
mass number 1. Atom number 1.
0n1 : neutron change 0.
Mass 1 amu.
-1e0 : electron change
–1. Mass negligible.
4. For an atom ‘X’, K, L and M
shells are completely filled. How many electrons will be present in it?
Answer:
28 [K=2, L=8, M=18]
5. What is the same about the
electron structures of:
a. Lithium, Sodium and Potassium.
b. Beryllium, Magnesium and
Calcium.
Answer:
a.
Lithium, sodium & potassium have 1 electron in their outermost shell.
b.
Beryllium, magnesium and calcium have 2 electrons in their outermost shell.
VII. Answer briefly:
1. How was it shown that atom has
empty space?
Answer: Rutherford
performed an experiment of bombarding a thin gold foil with very small positively
charged particles called α particles. He observed that most of the alpha
particles passed straight through the foil. He inferred by thin experiment that
most of the space in the atom is empty.
2. Why do 3517Cl and 3717Cl have the same chemical properties? In
what respect do these atoms differ?
Answer: 3517Cl, 3717Cl have same chemical properties because
they have same number of electrons but their atoms differ in the number of
neutrons.
3. Draw the structure of oxygen
and sulphur atoms.
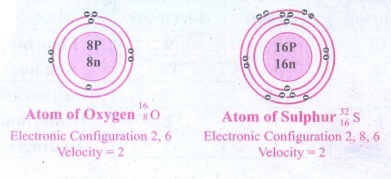
4. Calculate the number of
neutrons, protons and electrons :
(i) atomic number 3 and mass
number 7
(ii) atomic number 92 and mass
number 238.
Answer:
(i)
atomic number 3 and mass number 7
∴ No. of
electrons (or) No. of protons = 3
Mass number = No. of protons + No. of neutrons
∴ No. of
neutrons = 7 – 3 = 4
(ii)
atomic number 92 and mass number 238
Atomic number = 92
∴No. of
electrons = 92
No. of protons = 92
∴No. of
neutrons = 238 − 92 = 146
5. What are nucleons? How many
nucleons are present in Phosphorous? Draw its structure.
Answer:
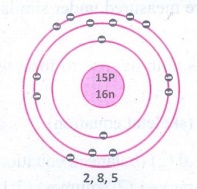
The
number of protons and neutrons present in the nucleus are called nucleons. The
number of nucleons present is phosphorus is 31.
VIII. Answer in detail
:
1. What conclusions were made
from the observations of Gold foil experiment?
Answer:
(i)
Atom has very small nucleus at the centre.
(ii)
There is large empty space around the nucleus.
(iii)
Entire mass of an atom is concentrated in a very small positively charged
region which is called the nucleus.
(iv)
Electrons are distributed in the vacant space around the nucleus.
(v)
The electrons move in circular paths around the nucleus.
2. Explain the postulates of
Bohr’s atomic model.
Answer:
The
main postulates are:
(i)
In atoms, the electron revolve around the nucleus in stationary circular paths
called orbits or shells or energy levels.
(ii)
While revolving around the nucleus in an orbit, an electron neither loses nor
gains energy.
(iii)
An electron in a shell can move to a higher or lower energy shell by absorbing
or releasing a fixed amount of energy.
(iv)
The orbits or shells are represented by the letters K,L,M,N,... or the numbers,
n = 1,2,3,4,....
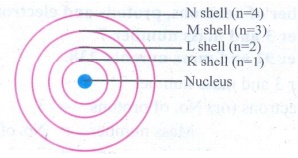
Energy levels
around the nucleus of an atom : Bohr’s model.
The
orbit closest to the nucleus is the K shell. It has the least amount of energy
and the electrons present in it are called K electrons, and so on with the
successive shells and their electrons. These orbits, are associated with fixed
amount of energy, so Bohr called them as energy level or energy shells.
3. State the Gay Lussac’s law of
combining volumes, explain with an illustration.
Answer:
Whenever
gases react together, the volumes of the reacting gases bear a simple ratio,
and the ratio is extended to the product when the product is also in gaseous
state, provided all the volumes are measured under similar conditions of
temperature and pressure.
Example:
Step
1: Hydrogen combines with oxygen to form water (word equation) Hydrogen +
Oxygen → Water.
Step
2: H2 + (1/2) O2 → H2O (skeletal equation)
Step
3: 2H2(g) + O2(g) → 2H2O (g) (balanced equation)
(2
Volumes) + (1 Volume) → (2 Volumes) (2:1:2)
This
law may be illustrated by the following example.
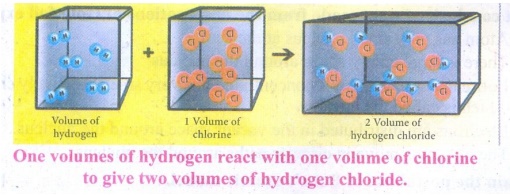
It
has been experimentally observed that two volumes of hydrogen reacts with one
volume of oxygen to form two volumes of water.
The
ratio of volume which gases bears is 2:1:2 which is a simple whole number
ratio.
Intext Activities
ACTIVITY - 1
Symbolically represent the
following atoms using atomic number and mass number.
a) Carbon
b) Oxygen
c) Silicon
d) Beryllium
Answer:
a)
Carbon – 126C
b)
Oxygen – 168O
c)
Silicon – 2814Si
d)
Beryllium – 94Be
ACTIVITY - 2
Assign the valency for
Phosphorus, Chlorine, Silicon and Argon
Answer:
Phosphorus
- P – 3.5
Chlorine
– Cl - 1
Silicon
- Si - 4
Argon
- Ar - 0
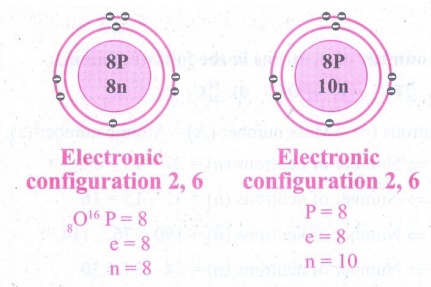
ACTIVITY - 4
Draw the structures of the
isotopes of oxygen O16 and O18. Atomic number of oxygen =
8.
Answer:
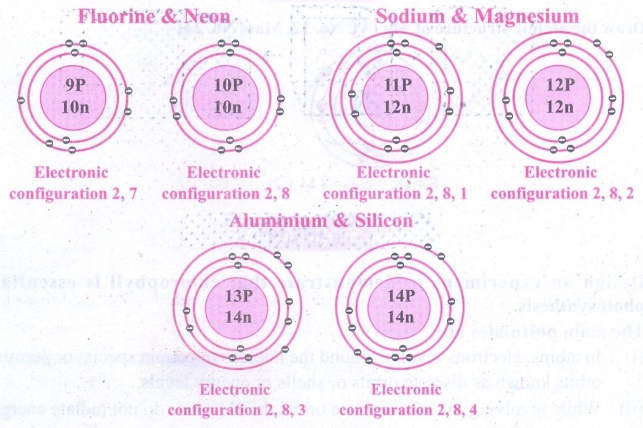
ACTIVITY - 5
Draw the model of the following
pairs of isotones:
(i) Fluorine & Neon
(ii) Sodium & Magnesium
(iii) Aluminum and Silicon.
Answer:
ACTIVITY - 6
Nitrogen combines with hydrogen
to form ammonia (NH3). Illustrate Gay Lussac’s law using this
example.
Answer:
Step
1: Nitrogen ↑ + Hydrogen ↑ → Ammonia ↑.
Step
2: N2 ↑ + H2 ↑ → NH3 ↑.
Step
2: N2 ↑ + 3H2 ↑ →
2NH3 ↑.
(1
volume ) + (3 volume ) → (2 volume )
1
volume of nitrogen reacts with 3 volumes of hydrogen to form 2 volumes of
ammonia gas. The ratio by volume which gases bear is 1 : 3 : 2.Which is a
simple while number ratio.
Test Yourself:
1. Calculate the number of neutrons
in the following atoms:
a) 2713Al
b) 3415P
c) 19076Os
d) 5424Cr
Answer:
a)
2713Al ⇒
Number of neutrons (n) = 27 – 13 = 14
b)
3415P ⇒
Number of neutrons (n) = 31 – 15 = 16
c)
19076Os ⇒
Number of neutrons (n) = 190 – 76 =
114
d)
5424Cr ⇒
Number of neutrons (n) = 54 – 24 = 30
Related Topics