Heat | Physics | Science - Answer the following questions | 9th Science : Physics : Heat
Chapter: 9th Science : Physics : Heat
Answer the following questions
HEAT
TEXT BOOK EXERCISES
IV. Answer briefly:
1. Define conduction.
Answer: The
process of transfer of heat in solids from a region of higher temperature to a
region of lower temperature without the actual movement of molecules is called
conduction.
2. Ice is kept in a double-walled
container. Why?
Answer: An
ice-box is made of double wall and the space between the walls is filled with
some non-conducting materials to provide heat insulation, so that the loss of
heat can be minimized. Hence ice is kept in a double-walled container.
3. How does the water kept in an
earthen pot remain cool?
Answer: As the
water seeps out of the earthern pot, it gets evaporated and takes away heat from
the vessel. The water in the pot gets cooled.
4. Differentiate convection and
radiation.
Answer:

Convection
•
The process of transfer of heat in which the heated molecules of a liquid (or
gas) themselves move to carry heat from the hot to the cold end is called
convection.
• Ex: Land and
sea breeze
•
Convection need matter to be present.
Radiation
•
The process of transfer of heat in which a material medium is not necessary and
heat is directly transferred from the hot body to the cold body is called radiation.
• Ex:
Transfer of heat energy from the sun.
•
Radiation can occur even in vacuum.
5. Why do people prefer wearing
white clothes during summer?
Answer: White
clothes absorb the least heat from the sun and hence keep us comfortable in
summer. On the other hand, dark coloured clothes absorb more heat from the sun
and keep us warm in winter.
6. What is specific heat
capacity?
Answer: Specific
heat capacity of a substance is defined as the amount of heat required to raise
the temperature of 1 kg of the substance by 1°C or 1 K.
7. Define thermal capacity.
Answer: Thermal
capacity is the heat required to raise the temperature of a unit mass of the
body by 1°C.
8. Define specific latent heat
capacity.
Answer: Specific
latent heat is the amount of heat energy absorbed or liberated by unit mass of
a substance during change of state without causing any change in temperature.
V. Answer in detail:
1. Explain convection in daily
life.
Answer:
Convection is the flow of heat through a fluid from places of higher
temperature to places of lower temperature by movement of the fluid itself.
Hot air balloons:
Air
molecules at the bottom of the balloon get heated by a heat source and rise. As
the warm air rises, cold air is pushed downward and it is also heated. When the
hot air is trapped inside the balloon, it rises.
Breeze:
During
day time, the air in contact with the land becomes hot and rises. Now the cool
air over the surface of the sea replaces it. It is called sea breeze. During
night time, air above the sea is warmer. As the warmer air over the surface of
the sea rises, cooler air above the land moves towards the sea. It is called
land breeze.
Chimneys:
Tall
chimneys are kept in kitchen and industrial furnaces. As the hot gases and
smoke are lighter, they rise up in the atmosphere.
2. What are the changes of state
in water? Explain
Answer:
(i)
The process of changing of substance from one physical state to another at a
definite temperature is defined as change of state.
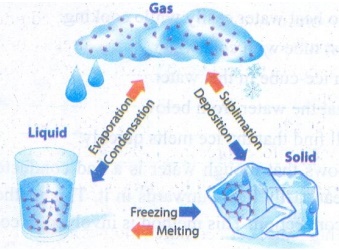
(ii)
For example, water molecules are in liquid state at normal temperature.
(iii)
When water is heated to 100°C, it becomes steam which is a gaseous state of
matter. On reducing the temperature of the steam it becomes water again.
(iv)
If we reduce the temperature further to 0°C, it becomes ice which is a solid
state of water. Ice on heating, becomes water again.
(v)
Thus, water changes its state when there is a change in temperature.
(vi)
The process in which a solid is converted to liquid by absorbing heat is called
melting or fusion.
(vii)
The process in which a liquid is converted to solid by releasing heat is called
freezing.
(viii)The
process in which a liquid is converted to vapor by absorbing heat is called
boiling or vaporization.
(ix)
The process in which a vapour is converted to liquid by releasing heat is
called condensation.
(x)
The process in which a solid is converted to gaseous state is called
sublimation.
3. How can you experimentally
prove water is a bad conductor of heat? How is it possible to heat water easily
while cooking?
Answer:
(a)
Half fill a test tube with cold water. Wrap a piece of ice in wire gauze and
drop it in the tube.
(i)
It will sink to the bottom.
(ii)
Now heat the top end of the test tube.
(iii)
The water soon begins to boil at the top but the ice below has still not fully
melted.
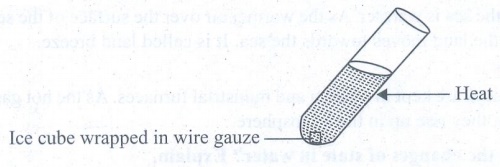
This
activity shows that water is a bad conductor of heat. It does not easily
conduct heat from the top to the bottom of the test tube.
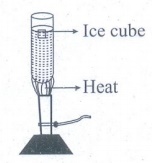
(b)
It is possible to heat water easily while cooking:
(i)
Fill a test tube with cold water.
(ii)
Drop an ice-cube in this water.
(iii)
Now heat the water from below.
(iv)You
will find that the ice melts quickly.
This
shows that though water is a bad conductor of heat, heat easily flows upwards
in it. This method of transfer of heat is called convection. This method is
involved in cooking.
VI. Numerical Problems.
1. What is the heat in joules
required to raise the temperature of 25 grams of water from 0°C to 100°C? What
is the heat in Calories?
(Specific heat of water = 4.18
J/g°C)
Given: Mass of
water m = 25g
Initial
temperature T1 = 0°C
Final
temperature T2 = 100°C
Change
in temperature ΔT = (T2 – T1)
= (100−0)° C ΔT = 100°C
Specific
heat of water C = 4.18 J/g°C
Solution:
Heat
required H (in joules) = m × c × ΔT
= 25 × 4.18 × 100
= 25×418
= 10450 J
Heat
required in calories = 1 calorie = 4.18 J
10450 J = 2497.60
calories
2. What could be the final
temperature of a mixture of 100 g of water at 90°C and 600 g of water at 20°C .
Given:
Mass of water m1 = 100g = 0.1 kg
Specific
heat capacity of water c = 4186 J
Temperature = 90°C
Mass of water m2= 600 g = 0.6 kg
Temperature = 20°C
Solution:
Heat
lost by hot water = Heat gained by cold water
m1 × c × θ1 = m2 × c × θ2
0.1×4186
× (90−TF) = 0.6×4186 × (TF−20)
0.1×
(90−TF) = (TF−20) × 0.6
9
− 0.1TF = 0.6TF – 12
0.7
TF = 21
TF
= 30°C
Final
temperature of a mixture = 30°C
3. How much heat energy is
required to change 2 kg of ice at 0°C into water at 20°C? (Specific latent heat
of fusion of water = 3,34,000J/kg, Specific heat capacity of water = 4200JKg−1K−1).
Given:
Mass of ice m
= 2kg
Specific latent heat of fusion of water = L = 3,
34,000 J/Kg
Change in temperature ΔT = (T2
– T1)
= (20 – 0)o
C
ΔT = 20oC
Specific
heat capacity of water C = 4200 J Kg-1 K-1
Heat Energy
required = m × c × ΔT + m × L
= 2 × 4,200 × 20 + 2 × 3,34,000
= 1,68,000 + 6,68,000
Heat energy required = 8,36,000 J
Intext Activities
ACTIVITY - 1
Take a glass of water and put
some ice cubes into it. Observe it for some time. What happens? The ice cubes
melt and disappear. Why did it happen? It is because heat energy in the water
is transferred to the ice.
Aim:
To
demonstrate transfer of heat.
Material required:
A
glass of water, ice cubes.
Procedure:
Take
a glass of water and put some ice cubes into it. Observe it for some time. What
happens?
Observation:
The
ice cubes melt and disappear. It is because heat energy in the water is
transferred to the ice.
Conclusion:
Heat
transfer takes place when heat energy flows from the object of higher
temperature to an object with lower temperature.
ACTIVITY - 2
Take metal rods of copper,
aluminum, brass and iron. Fix a match stick to one end of each rod using a
little melted wax. When the temperature of the far ends reach the melting point
of wax, the matches drop off. It is observed that the match stick on the copper
rod would fall first, showing copper as the best conductor followed by
aluminum, brass and iron.
Aim:
To
compare the conducting powers of various metals .
Materials required:
Metal
roads of copper, aluminium, brass and iron, match stick, melted wax.
Procedure:
Fix
a match stick to one end of each rod using the little melted wax. When the
temperature of the far ends reach the melting point of wax, the matches drop.
Observe what happens?
Observation:
The
match stick on the copper rod would fall first, showing copper as the best
conductor followed by aluminum, brass and then iron.
Conclusion:
Metals
are good conductors of heat. Copper is the best conductor of heat.
ACTIVITY-3
Drop a few crystals of potassium
permanganate down to the bottom of a beaker containing water.When the beaker is
heated just below the crystals, by a small flame, purple streaks of water rise
upwards and fan outwards.
Aim:
To
demonstrate transfer of heat through convection in liquids .
Materials required:
Crystals
of potassium permanganate, beaker containing water .
Procedure:
Drop
a few crystals of potassium permanganate down to the bottom of a beaker
containing water, heat it by a small flame.
Observation:
When
the beaker is heated, just below the crystals purple streaks of water rise
upwards and fan outward.
Conclusion:
Water
molecules at the bottom of the beaker receive heat energy and move upward and
replace the molecules at the top.
This
activity shows that the flow of heat through a fluid from places of higher temperature
to places of lower temperature by movement of the fluid itself.
ACTIVITY - 4
Take some crushed ice cubes in a
beaker and note down the temperature using thermometer. It will be 0°C. Now heat
the ice in the beaker. You can observe that ice is melting to form water.
Record the temperature at regular intervals and it will remain at 0°C until
whole ice is converted to liquid. Now heat the beaker again and record the
temperature. You can notice that the temperature will rise up to 100°C and it
will retain the same even after continuous heating until the whole mass of
water in the beaker is vaporized.
Aim:
To
understand latent heat of water
Materials Required:
Crushed
ice cubes, beaker and thermomerter.
Procedure:
Take
some crushed ice cubes in a beaker and note down the temperature using
thermometer. It will be 0°C. Now heat the ice in the beaker, (i) Observe and
record the temperature at regular intervals. Heat the beaker again and record
the temperature.
Observation:
(i)
Ice is melting to form water.
(ii)
Water will remain at 0°C until whole ice is converted to liquid.
(iii)
On further heating, we can observe that the temperature will rise up to 100°C
and the temperature will be at 100°C even after continuous heating until the
whole mass of water in the beaker is vapourized.
Conclusion:
In
this activity, the temperature is constant at 0°C until entire ice is converted
into liquid and again constant at 100°C until all the water is converted into
vapour. It is because, when a substance changes from one state to another, a
considerable amount of heat energy is absorbed or liberated. This energy is
called latent heat.
Related Topics