Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Fishes as social animals: aggregation, aggression, and cooperation
Agonistic interactions of Fishes
Agonistic interactions
Aggressive interactions usually result from competition or potential competition for valuable resources. Defendable resources include food and feeding areas, refuge and resting sites, mates and mating grounds, eggs, and young. Defense can produce dominance hierarchies in aggregating fishes or territoriality in more solitary species. In addition, a hierarchy can exist among neighboring territory holders, and dominant–subordinate relationships often exist when solitary fish meet.
Behavioral hierarchies
Dominance hierarchies (“peck orders”) are either linear or despotic. In linear hierarchies, an alpha animal dominates all others, a beta animal is subordinate to the alpha but dominates lower ranked individuals, etc., down to the last, or omega, individual. Such a hierarchy exists in harems of the sex-changing Cleaner Wrasse, Labroides dimidiatus. A single male dominates up to six females, which in turn have their own linear hierarchy. Linear hierarchies also exist in salmonids, several livebearers, and centrarchid sunfishes (Gorlick 1976). In despotic situations, a single individual, the despot, is dominant over all other individuals, while subordinate animals have approximately equal ranks. In captive anguillid eels, a single large individual can monopolize 95% of a 300 L aquarium, relegating 25 other individuals to the remaining area where they mass together in continual contact. Despotic hierarchies have also been observed in Coho Salmon, Oncorhynchus
kisutch, and bullhead catfishes, Ameiurus spp. (Paszkowski & Olla 1985).
Dominance can be determined by size, sex, age, prior residency, and previous experience. In general, large fish dominate over smaller, older over younger, and residents over intruders. In many species, males usually dominate females, whereas in others, such as Guppies, females dominate males. Previous experience, in terms of recent wins and losses, often determines the outcome of future interactions; victorious fish tend to be aggressive and defeated fish submissive. Dominant fish typically occupy the most favorable microhabitats, relegating subordinates to suboptimal sites with respect to cover availability, current velocity, or prey densities. As a consequence, dominant individuals will have higher feeding rates, which ultimately lead to faster growth, better condition, and higher fitness (e.g., salmonids; Bachman 1984; Gotceitas & Godin 1992). Dominance hierarchies have also been observed in requiem and hammerhead sharks, minnows, ictalurid catfishes, amblyopsid cavefishes, cods, ricefishes (Oryziidae), topminnows, livebearers, centrarchid sunfishes, cichlids, labrids, blennies, and boxfishes (Ostraciidae).
Territoriality
Territoriality implies a defended space, either the personal space around an individual (=individual distance) or a bounded area around some resource (e.g., Grant 1997). A territory may encompass several resources, as in male pomacentrid damselfishes in which the territory provides food (algae), a spawning site and the eggs spawned there, and refuge holes from predators where the territory holder also rests at night. Territories are often subunits of the larger home range occupied by an individual (see next section).
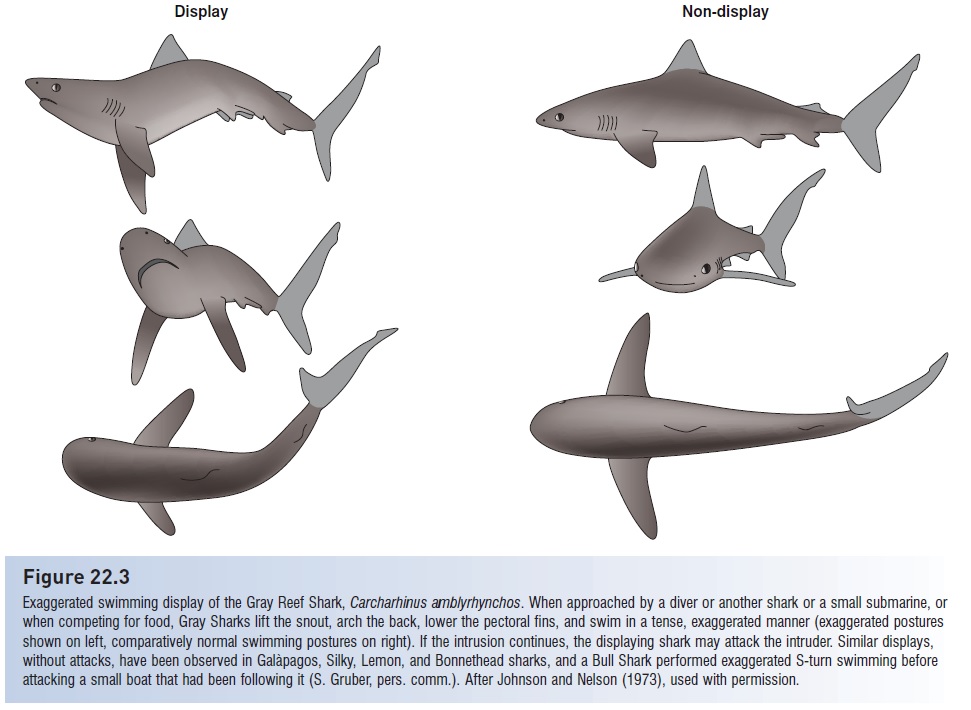
Territoriality is widespread in fishes, occurring in such diverse groups as anguillid eels, cyprinids, ictalurid catfishes, gymnotid knifefishes, salmonids (affecting stocking programs and the effects of introduced species), frogfishes, sticklebacks, pupfishes, rockfishes, sculpins, sunfishes and black basses, butterfl yfishes, cichlids, damselfishes, wrasses barracuda, blennies, gobies, surgeonfishes, and anabantids. Territoriality has not been observed in agnathans or elasmobranchs, although the threat responses of Gray Reef Sharks (see Fig. 22.3) may represent defense of personal space. Territorial defense often involves displays such as fin and gill spreading, lateral displays and exaggerated swimming in place, vocalizations, chasing, and finally biting. Prolonged exchanges of displays frequently occur at territorial boundaries. When territories are being established or contested (as opposed to temporary trespassing), priority of ownership, previous experience, and individual size usually determine the outcome of a dispute. Again, territory holders win over intruders, previous winners defeat previous losers, and large fish win over small fish. Territories near one another can create “territorial mosaics” of several contiguous territories (e.g., salmonids, pomacentrids, mudskippers, blennies; Keenleyside 1979).
The costs of territorial defense – energy and time expended, exposure to predators, resource loss to competitors while defending distant portions of a territory – increase with increasing territory size. Food production affects territory size because a territorial animal must often meet its daily energy requirements from the resources available within its territory. As would be expected, increased food density leads to a decrease in territory size (e.g., in Rainbow Trout; rockfishes, Scorpaenidae; surfperch, Embiotocidae; several damselfishes; Hixon 1980a, 1980b). Interestingly, in Beau Gregory Damselfish, males decrease territory size with increasing food but females respond by increasing territory size. Larger females can produce more eggs and hence increased energy intake apparently overcomes the costs of defending a larger territory (Ebersole 1980).
Territoriality is often flexible. Territorial boundaries and intensity of defense can vary as a function of the relative impacts of different intruders. Herbivorous damselfishes defend a larger space against large competitors such as parrotfishes and surgeonfishes than against small damselfishes; damselfishes also tolerate large competitors for shorter times inside the territory, attacking them more aggressively. The strongest attacks are directed at potential egg predators, which have the greatest relative impact on the reproductive success of the damselfish. Juvenile Coho Salmon also defend a larger territory against larger conspecific intruders. Butterfl yfishes chase species with which they overlap in diet but tolerate the presence of non-competitors (Myrberg & Thresher 1974; Reese 1975; Ebersole 1977; Dill 1978).
Territoriality may also vary over time at several levels. Juvenile grunts (Haemulidae) form daytime resting shoals over coral heads. Individuals stake out small territories of about 0.04 m2 within the shoal; the territories often contain refuge sites from predators. These territories are defended vigorously with open mouth displays, chases, and biting. In the evening, the shoal becomes a polarized school that moves from the reef to adjacent grassbeds to feed. No agonism is seen during the migratory period, as such behavior would negate the antipredator function of the school when moving across the dangerous reef edge. Once in the grassbeds, the shoals break up and the fish occur as widely spaced, foraging individuals, implying space-enforcing behaviors (McFarland & Hillis 1982). Territoriality changes with age in many species. Young Atlantic Salmon are territorial in streams. As they grow and their food requirements shift to larger prey, they move into deeper water and join foraging groups that have dominance hierarchies rather than territories (Wankowski & Thorpe 1979). In some species, agonistic interactions occur during the breeding season, with fish aggregating peaceably at other times (e.g., codfishes).
Home ranges
Territories are usually a spatial subset of the larger area that a fish uses in its daily activities. Such home ranges or activity spaces are common in fishes, which move over the same parts of the habitat at fairly predictable intervals, often daily but also at other timescales (Lowe & Bray 2006). Home range is dependent on fish size and species. Larger species and individuals generally move over larger ranges, although range size may decrease with growth in an individual (e.g., Bocaccio Rockfish, Sebastes paucispinis, Starr et al. 2002; Greasy Grouper, Epinephelus tauvina, Kaunda- Arara & Rose 2004). Home range may be very restricted, as in the few square meters around a coral head (e.g., gobies, damselfishes) or contained in a tide pool (e.g., pricklebacks) (Sale 1971; Horn & Gibson 1988; Kroon et al. 2000). Some benthic stream fishes may have ranges of 50–
100 m2 (Hill & Grossman 1987) whereas others may range over hundreds of meters (e.g., Albanese et al. 2004). Intermediate ranges of a few hundred square meters characterize many lake, riverine, kelpbed, and reef species, although many large coral reef fishes are relatively sedentary, utilizing concentrated reef resources. Home ranges of many large species such as groupers and snappers may not exceed 0.1 km2 (Pittman & McAlpine 2003). Pelagic predators such as tunas, salmons, large sharks, and billfishes cross entire oceans seasonally or repeatedly. But even these oceanic wanderers show evidence of periodic residence in certain areas on a seasonal basis (see Annual and supra-annual patterns: migrations).
Although a fish may spend 90% of its time each day within its home range, it is common to encounter individuals many meters or even kilometers away from their usual activity space. Such movements characterize fishes in most habitats (e.g., Fausch et al. 2002). These periodic excursions imply a well-developed homing ability in many species. Numerous studies, involving experimental displacements of tagged individuals, have repeatedly shown a strong tendency to return to home sites in many fishes. The Tidepool Sculpin, Oligocottus maculosus, can be displaced as far as 100 m from its home tide pool and will find its way back, using either visual or chemical cues. Older fish can still remember the way home after 6 months in captivity. Younger fish have shorter memory spans and require both visual and chemical cues to find home successfully, whereas older individuals do not require both types of information (Horn & Gibson 1988). In some species, adults find their way around the home range by identifying landmarks, creating a cognitive map of the locale (Reese 1989). In general, older fish have a stronger homing tendency and often occupy smaller home ranges than younger individuals. Since juveniles are the colonists that most often invade recently vacated or newly created habitat, this generalization is not surprising (Gibson 1993).
The use of a home range is affected by several components of a fish’s biology. Normal ranges are often deserted during the breeding season. This may involve no more than a female damselfish having to leave her territory to lay eggs in the adjacent territory of a male, but can also involve long-distance movements of 100 km or more to traditional group-spawning areas, such as occurs in many seabasses on coral reefs. Colorado Pikeminnows, Ptychocheilus Lucius (Cyprinidae), make annual round-trip movements of as much as 400 km between traditional spawning and normal home range areas. The home range also interacts with shoaling behavior in some species and can differ among individuals within a species. Yellow Perch, Perca flavescens, form loose shoals (see next section) of many individuals that forage in the shallow regions of North American lakes. Home range size is directly correlated with the amount of time individuals spend in shoals. Individuals with strong shoaling tendencies also have larger home ranges. As a shoal enters the residence area of an individual, the resident fish joins the shoal until the shoal moves to the boundary of the home range. Home ranges and fi delity to particular sites have probably arisen because intimate knowledge of an area increases an individual’s ability to relocate productive feeding areas or effective refuge and resting sites, reducing the amount of energy expended and risk incurred while searching for such locales (Helfman 1984; Tyus 1985; Shapiro et al. 1993).
Knowledge of home range size has important implications for fish conservation, especially with regard to the creation of reserves and protected areas. Reserves must be large enough to encompass the home ranges of both sed entary and mobile species; without specific knowledge of daily, seasonal, and ontogenetic movements, a reserve might fail to encompass the range of habitats or the actual areal expanse needed to protect most species and most life history stages (Kramer & Chapman 1999; Cooke et al. 2005; Sale et al. 2005). The likelihood of spillover of individuals into adjacent areas, an anticipated benefit of reserve creation, also depends on movement and will vary in relation to reserve design and species behavior. Relationships between home range size and reserve design have been examined in tropical and temperate locales involving taxa as diverse as seabasses, sparids, goatfishes, wrasses, and surgeonfishes, to name a few (Meyer et al. 2000; Egli & Babcock 2004; Meyer & Holland 2005; Popple & Hunte 2005; Topping et al. 2005).
Related Topics