Chapter: Modern Pharmacology with Clinical Applications: β-Lactam Antibiotics
β-Lactam Antibiotics: Mechanism of Action
MECHANISM OF
ACTION
The final reaction in
bacterial cell wall synthesis is a cross-linking of adjacent peptidoglycan
(murein) strands by a transpeptidation reaction. In this reaction, bacter-ial
transpeptidases cleave the terminal D-alanine from a pentapeptide on one
peptidoglycan strand and then cross-link it with the pentapeptide of another
peptido-glycan strand. The cross-linked peptidoglycan (murein) strands give
structural integrity to cell walls and permit bacteria to survive environments
that do not match the organism’s internal osmotic pressure.
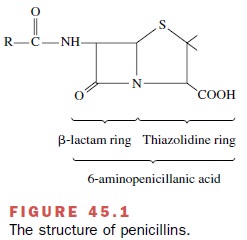
The β-lactam antibiotics
structurally resemble the terminal D-alanyl-D-alanine (D-Ala-D-Ala) in the pen-tapeptides on peptidoglycan
(murein) (Fig. 45.1). Bacterial transpeptidases covalently bind the β-lactam
antibiotics at the enzyme active site, and the resultant acyl enzyme molecule
is stable and inactive. The intact β-lactam ring is required for antibiotic
action. The β-lactam ring modifies the active serine site on transpep-tidases
and blocks further enzyme function.
In addition to transpeptidases, other penicillin-bind-ing proteins (PBPs) function as transglycosylases and carboxypeptidases. All of the PBPs are involved with assembly, maintenance, or regulation of peptidoglycan cell wall synthesis. When β-lactam antibiotics inactivate PBPs, the consequence to the bacterium is a structurally weakened cell wall, aberrant morphological form, cell lysis, and death.
Related Topics