Chapter: Civil : Construction Materials: Modern Materials
Glass
Glass
Glass is an amorphous substance
having homogeneous texture. It is a hard, brittle, transparent or translucent
material. It is the most common material glazed into frames for doors, windows
and curtain walls. The most common types used in building construction are
sheet, plate, laminated, insulating, tempered, wired and patterned glass. Most
ordinary colourless glasses are alkali-lime silicate and alkali-lead silicate
with tensile and compressive strengths of about 30-60 N/mm2
and 700-1000N/mm2,
respectively and modulus of elasticity in the range 0.45 × 10 5 to
0.8 × 10 5 N/mm2. The strength is very much afftected by
internal defects, cords and foreign intrusions. The main shortcoming of glass
is its brittleness which depends on a number of factors, the chief one being
the ratio of the modulus of elasticity of the material to its tensile strength.
Constituents
The raw materials used in
manufacturing glass are sand, lime (chalks) and soda or potash which are fused
over 1000 o C. Oxides of iron, lead and borax are added to modify hardness,
brilliance and colour. The functions of the various ingredients are as follows.
Silica is used in the form of
pure quartz, crushed sandstone and pulverised flint; should be free from iron
contents for best quality glass. Since it melts at very high temperatures
(1710 o C) carbonates of sodium or potassium are added to lower down the fusing
temperature to about 800 o C. These also make liquid silica more viscous and
workable.
Lime is used in the form of
limestone, chalk or pure marble and sometimes marl. The addition of lime makes
the glass fluid and suitable for blowing, drawing, rolling, pressing or
spinning. It also imparts durability and toughness to glass. Excess of lime
makes the molten mass too thin for fabrication.
Soda acts as an accelerator for the fusion of
glass and an excess of it is harmful.
Potash renders glass infusible and makes glass fire
resistant.
Lead Oxide imparts colour,
brightness and shine. When 15-30% of it added to
substitute lime it lowers the melting point, imparts good workability, while
its transparency is lost with the glass becoming brittle and crystalline.
Cullets are broken glasses added
to act as a flux to prevent loss of alkali by volatisation during the process
of forming glass and also to lower the fusion temperature. However, flux may
reduce the resistance of glass to chemical attack, render it water-soluble or
make it subject to partial or complete devitrification (crystallisation) on
cooling. These crystalline areas are extremely weak and brittle. Stabilizers
are added to overcome these defects.
Titanic
acid, oxides of Nickel and Cobalt are used for chromatic neutralisation.
Note: Iron is not desirable as a
constituent. However, when present it imparts a bottle
green colour to the glass. To overcome this manganese dioxide
known as glass maker's soap is added which washes the
liquid glass and removes the colour.
Manufacture
Glass is manufactured in the following four steps:
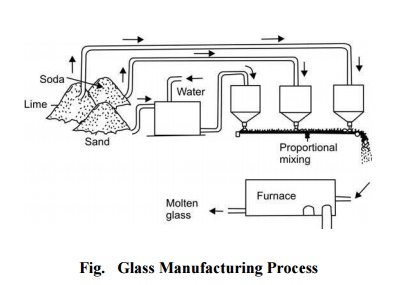
Melting The raw materials - lime,
soda and sand - separately
cleaned, ground, sieved
(called 'Batch') in
definite proportion and mixed with water are fused in a continuous type
(tank) furnace or batch-type (pot) furnace. The charge in the first stage
melts, forming a bubbly, sticky mass, and as the temperature is raised (1100 o C-1200 o C)
it turns to a more watery liquid and the bubbles rise to the surface. The
melting process in case of ordinary soda-glass involves the following series of
reactions:
CaCO3+SiO2
¾® CaSiO3 + CO2-
Na2CO3
+ SiO2 ¾® Na 2SiO3
+ CO2-
When all
the carbon dioxide has escaped out of the molten mass, decolourisers such as
MnO2 or nitre are added to do away with ferrous compounds and
carbon. The colouring salts are added at this stage. Heating is continued till
the molten mass is free from bubbles and glass balls. As the glass cools (800 o
C), it is ready to be drawn or floated to its desired thickness and size at the
other end of the furnace as shown by a flow diagram in Fig..
Forming
and Shaping The molten glass can be fabricated to desired
shapes by any one of the following methods:
Blowing A 2 m long and 12 mm
diameter blow pipe is dipped in the molten glass and taken out. It is held
vertically and is vigorously blown by the operator. The sticking molten glass
takes the shape of a hollow ball. On cooling it is
reheated and the blowing
operation repeated a number of times till the desired articles are ready.
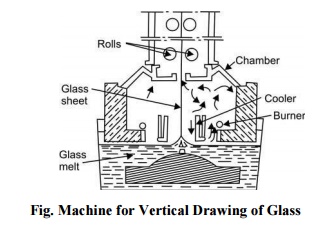
Flat
Drawing The process of drawing the glass up into a sheet begins when an grille
(bait) is lowered into the glass in the kiln. In a short time the liquid molten
glass adheres to the bait, and as the bait is slowly lifted it draws a sheet of
glass. The bait and the drawn sheet of glass are then drawn through rollers,
the bait is cracked off and a continuous sheet of glass is drawn up. This sheet
is then slowly cooled in a chamber and annealed for cutting into proper size. A
machine for vertical drawing of glass is shown in Fig..
Compression Moulding In this
process moulds are used to obtain the articles of desired shapes.
Spinning A machine is used to
spin the molten glass. The fibres so produced are very fine and are used for
heat and sound insulation.
Annealing Glass articles are
allowed to cool under room temperature by passing through different chambers
with descending temperature. If cooled rapidly, the glass being bad conductor
of heat, the superficial layer cools down first and strain develops in the
interior portions, which causes unequal expansion and the articles are likely
to crack.
Finishing
After annealing the glass articles are cleaned, ground, polished, cut and sand
blasted.
Classification
Depending upon the constituents
glasses are classified as soda-lime glass, lead glass and boro-silicate glass.
Soda-lime Glass is also
known as soda-ash glass, soda glass or soft glass. Soda-lime glass is obtained
by fusing a mixture of silica, lime and soda. The quality of this glass can be
improved by adding alumina and magnesium oxide and the glass is then called
crown glass. This is the most common type of glass used in doors, windows and
for making glass-wares such as bottles.
Lead Glass also
known as flint glass is obtained by fusing a mixture of silica, lead and
potash. It is free from iron impurities and is colourless. Lead glass
has high shining appearance and can take polish. It is not affected by
temperature. Electric bulbs, optical glasses, cut glass, ornamental glass works
and radio valves are some of the articles made from it.
Boro-silicate Glass is
obtained by fusing a mixture of silica, borax, lime and felspar. The examples
are pyrex glass and heat resisting glass. Boro-silicate glass can withstand
high temperatures and is most suitable for making laboratory equipments and
cooking utensils.
Commercial Forms
Sheet Glass is used
for glazing doors, windows and partitions and is obtained by blowing the molten
glass into the shape of a cylinder. The ends of the cylinder so produced are
cut away and the cylinder is flattened over a plane tray. It is available in
thicknesses of 2, 2.5, 3, 4, 5, 5.5 and 6.5 mm and up to 1750 × 1100 mm size
and is classified as
Type Uses
Ordinary
glazing quality General
engineering purpose
Selected
glazing quality Class
works
Special
selected quality Superior
quality works such as show cases
and
cabinets etc.
Plate Glass is used
for all engineering purposes and is superior to sheet glass. A plate glass differs
from a sheet glass in that it has a parallel, distortion-free surface obtained
by grinding or floating process. It is produced by pouring the molten glass on
casting tables and levelling it to an uniform thickness. Both the glass
surfaces are then ground, smoothened and polished. Glass so produced is clear
and contains unblemished true plane surfaces and is available in thicknesses of
3 to 32 mm and sizes up to 2750 × 900 mm. It is classified as
Type Uses
Ground
glass quality Showcases,
cabinets, counters, shop fronts, etc.
Selected
glazing quality Making
mirrors
Special
selected quality High
class works, wind screen of vehicles
Tempered
Glass is made from plate glass by reheating and sudden cooling and
is 3 to 5 times stronger than plate glass. Although not unbreakable, it
resists bending stress better than plate glass and, when broken, the pieces are
relatively small in size. It is used extensively in sports arenas, sliding
doors and curtain walls.
Wired Glass is
produced by embedding wire nets 0.46 to 0.56 mm into the centre of sheet glass
during casting. The minimum thickness of wired glass is 6 mm. When broken
it does not fall into pieces. It has higher melting point than ordinary glass.
Wired glass is used for fire resisting doors and windows, for sky lights and
roofs. A special example of this is wired-refrax glass which transmits 100 per
cent more light than the other glasses.
Obscured Glass is made
comparatively opaque to sunlight. Also known as patterned glass. They are
classified as frosted, rolled and ribbed.
Frosted glass is produced by
subjecting the polished face of the glass to a sand blast which grinds off the
surface. It can also be produced by etching on glass by hydrofluoric acid.
Rolled glass has a series of waves
of desired pattern on the surface and is also known as figured rolled glass.
Ribbed glass A series
of triangular ribs are produced in the glass during casting.
Laminated Glass is made
by sandwiching a layer of polyvinyl butyral between two or more layers
of plate or sheet glass. It is also lso known as safety glass. The examples are
heat proof glass, sound proof glass and bullet proof glass. Heat and sound
proof glasses Two or more glass plates are sandwiched by a tinted plastic inner
layer. It provides high resistance to heat and glare. By increasing the
thickness of plastic layer the glass can be made more sound resistant.
Bullet proof glass is produced by
placing vinyl plastic and glass in several alternate layers and pressing them
with outer layers of glass. It is used in banks, jewellery stores and display
windows.
Insulating glass is composed of
two glass plates into which a layer of 6-13 mm
thick dehydrated air is sealed. The round edges are formed by fusing together
the two glass plates. These glasses reduce the heat transmission by 30-60 per
cent.
Heat absorbing Glass is bluish
green in colour and cuts ultra violet rays of sun. The example is calorex.
It is used in railway carriages, factories, hospitals, health clubs and
kitchens.
Ground Glass In this
type of glass one face of plate or sheet glass is made rough by grinding. It
is used for maintaining privacy by obstructing vision and at the same time
allowing light. The ground glass is used for bedrooms, toilets and for making
black boards.
Block Glass is hollow
sealed made by fastening together two halves of pressed glass. It is used for
making partitions.
Coloured Glass is produced by adding
oxides of metals to molten glass:
Types of glasses Metal
oxide
Lead
glass, 1 per cent of cupric oxide and 1
Ruby red glass per
cent of
magnetic oxide of iron
Ruby rose glass Gold
chloride is used as colouring agent.
Brownish
red colour is obtained by adding
oxide
of
iron, bluish red shade is obtained by
Adding
2
per cent MnO2 and -4 per cent nitre
(KnO3).
0.1
per cent of cobalt oxide in ordinary
Blue glass glass.
Yellow glass 2-3% of alkali uranate.
(a) Uranium glass (greenish yellow)
(b) Selenium glass (orange) Selenite and a
reducing agent or ferric oxide
Green glass (emerald green) and MnO2.
Oxide
of chromium Cr2O7.
Violet glass (violet) MnO2
Black glass Oxide of Co and Mn.
Opal Glass is also
known as milk glass. It is produced by adding bone ash, oxide of tin and white
arsenic to vitreosil (99.5% silica glass known as clear silica glass). The
composition is 10 parts of sand, 4 parts cryolite and 1 part zinc oxide.
Enamel Glass is
produced by adding calcined lead and tin oxide to the ordinary glass. The composition
is 10 parts sand, 20 per cent lead and tin oxide and 8 parts potash.
Optical
Glass contains phosphorus, lead silicate and a little cerium oxide,
the latter capable of absorbing ultraviolet light injurious to eyes.
They are used for making lenses.
Related Topics