Chapter: Biochemistry: Practicals
Estimation of Calcium (Titrimetric Method)
ESTIMATION
OF CALCIUM (TITRIMETRIC METHOD)
Aim
To estimate the amount of calcium present in the
given serum sample.
Principle
Calcium is precipated as calcium oxalate with
ammonium oxalate. The precipate is washed with ammonia to remove the chloride
ions. The washed precipate is then made to react with 1N sulphuric acid. The
liberated oxalic acid is now estimated by titrating against standardised
potassium permanganate.The amount of oxalic acid liberated is proportional to
the amount of calcium.
Reagents Required
1. Ammonium oxalate solution (4 %)
4g of ammonium oxalate dissolved in 100ml of
distilled water.
2. Ammonia solution (2%)
2ml of ammonia of specific gravity 0.88 is
diluted to 100ml with distilled water.
3. Potassium permanganate (0.1N)
This is prepared by dissolving 3.16 g of
potassium permanganate in 1 litre of distilled water.
4. Standard oxalic acid solution (0.1N)
It is prepared by dissolving 630mg of oxalic
acid in 100ml of distilled water.
5. Sulphuric acid (1N)
Procedure
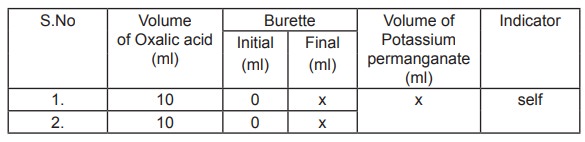
Standardisation of Potassium permanganate
10ml of oxalic acid is pipetted out into a clean
conical flask and 10ml of dilute sulphuric acid is added and heated to 60 ° C.
It is titrated against potassium permanganate in the burette. The end point is
the appearance of pale permanent pink colour. Titrations are repeated for
concordant values.
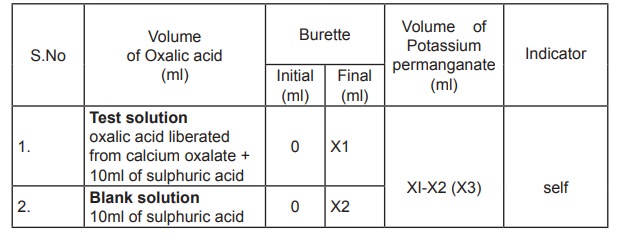
Precipitation of calcium oxalate
2ml of serum is taken in a centrifuge tube and
2ml of distilled water is added followed by 1ml of 4 % ammonium oxalate. The
contents are mixed and allowed to stand overnight at 4 ° C for complete
precipitation of calcium. The precipate is separated by centrifugation and the
supernatant is discarded. To the precipitate 3ml of 2% ammonia is added and
centrifuged. This procedure is repeated thrice and the supernatant is tested
for the presence of chloride. 10 ml of 1N sulphuric acid is added and warmed
for solubilisation. This solution is now titrated against potassium
permanganate and the volume consumed is noted.
10 ml of 1N sulphuric acid is treated as blank
and titrated against potassium permanganate. The end point is the appearance of
pale permanent pink colour. Titrations are repeated for concordant values and
the amount of calcium present is then calculated.
Tabular
Column
Titration I
Standardisation of Potassium permanganate
Standard Oxalic acid Vs Potassium permanganate
Volume of oxalic acid V1 =
10ml
Normality of oxalic acid.N1 = 0.1N
Volume of potassium permanganate V2 = x ml
Normality of potassium permanganate N2 =?
Normality of potassium permanganate N2 =
V1 N1/ V2
=Y
Titration
II
Estimation of Calcium in serum
Calculation
The amount of calcium present in the given
sample can be calculated by using the equation
1ml of 0.1N potassium permanganate is equivalent
to 0.2 mg of calcium Therefore, X3 ml of' Y' N potassium permanganate is
equivalent to
0.2 x X3 xY / 1 x 0.1 = Z mg of calcium.
2ml of serum contains Z mg of calcium
Therefore, 100 ml of serum contains 100 x Z / 2
mg of calcium
Result
The amount of calcium present in the given serum
sample is_____mg/100ml
Related Topics