Chapter:
Testing Of Cement: Physical Tests (IS: 4031)
Testing Of Cement: Physical Tests (IS: 4031)
Fineness Test
The degree of fineness of cement
is the measure of the mean size of the grains in it. There are three methods
for testing fineness: the sieve method-using 90
micron (9 No.) sieve, the air permeability method- Nurse
and Blains method and the sedimentation method- Wagner
turbidimeter method. The last two methods measure the surface area, whereas the
first measures grain size. Since cement grains are finer than 90 micron, the
sieve analysis method does not represent true mean size of cement grains. Also,
the tiny cement grains tend to conglomerate into lumps resulting in distortion
in the final grain size distribution curves. Considering these demerits,
fineness is generally expressed in terms of specific area, which is the total
surface area of the particles in unit weight of material.
Conditions Affecting Fineness:
The chemical composition and the degree of calcination influence the hardness
of the clinker and consequently the fineness to which the cement is ground.
Clinker, high in iron or silica, is apt to be hard and difficult to grind. The
same is true with a hard-burned clinker. Fineness is also influenced by the
time of grinding and the character of the pulverizing machinery. It has been
found that cement becomes finer with age provided it does not absorb too much
moisture. This is probably due to the decrepitation of the coarser grains
resulting from the hydration of the embedded lime particles.
Importance: Finer the cement,
more is the strength since surface area for hydration will be large. With
increase in fineness, the early development of strength is enhanced but the
ultimate strength is not affected. An increase in the fineness of the cement
increases the cohesiveness of the concrete mix and thus reduces the amount of
water which separates to the top of a lift (bleeding), particularly while
compacting with vibrators. However, if the cement is ground beyond a certain
limit, its cementative properties are affected due to the prehydration by
atmospheric moisture. Finer cement reacts more strongly in alkali reactive
aggregate. Also, the water requirement and workability will be more leading to
higher drying shrinkage and cracking.
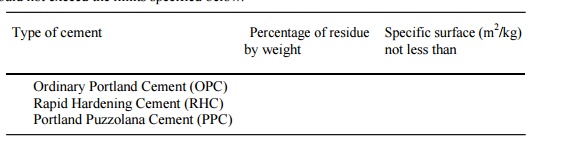
Sieve
Method: 100 g of cement sample is taken and air-set lumps, if any,
in the sample are broken with fingers. The sample is placed on a 90
micron sieve and continuously sieved for 15 minutes. The residue should not
exceed the limits specified below:
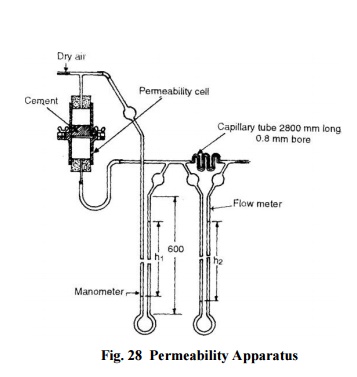
Air Permeability Method: The
fineness of cement is represented by specific surface, i.e. total surface area
in cm2 per gram or m2 per kilogram of cement and is
measured by Lea and Nurse apparatus or by wagner turbidimeter..
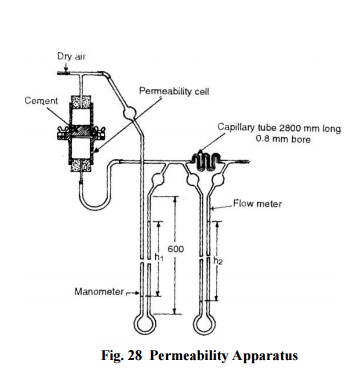
The Lea
and Nurse apparatus shown in Fig. 28 essentially consists of a permeability
test cell-where cement is placed and air pressure is
applied, flowmeter-to determine the quantity of air passing
per second through its capillary tube per unit difference of pressure, and
manometer-to measure the air pressure.
To
determine the fineness, a cement sample of 20 mm height is placed on a
perforated plate (40 micron perforations) and air pressure is applied. The
manometer is connected to the top of the permeability cell and the air is
turned on. The lower end of the permeability cell is then slowly connected to
the other end of the manometer. The rate of flow is so adjusted that the
flowmeter shows a pressure difference (h2) of 30-50 cm. The reading
(h 1) in the manometer is recorded. The process is repeated till the
ratio h1/h2 is constant. The specific surface is given by
the expression
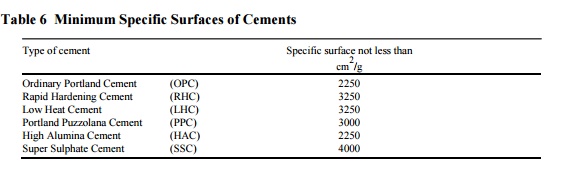
The minimum specific surface for various cements should be as
specified in Table 6.
Table 6 Minimum Specific Surfaces of Cements
Wagner Turbidimeter Method: L.A.Wagner
developed a turbidimeter to estimate the surface area of one gram of
cement. The cement is dispersed uniformly in a rectangular glass tank filled
with kerosene. Then, parallel light rays are passed through the solution which
strike the sensitivity plate of a photoelectric cell. The turbidity of the
solution at a given instant is measured by taking readings of the current
generated by the cell. By recording the readings at regular intervals while the
particles are falling in the solution, it is possible to secure information
regarding the grading in surface area and in size of particle. Readings are
expressed in sq. cm per gram.
Consistency Test
This is a test to estimate the quantity of mixing water to
form a paste of normal consistency defined as
that percentage water requirement of the cement paste, the
viscosity of which will be such that the
Vicat's plunger penetrates up to a point 5 to 7 mm
from the bottom of the Vicat's mould.
Importance: The water requirement
for various tests of cement depends on the normal consistency of the cement,
which itself depends upon the compound composition and fineness of the cement.
Test Procedure: 300 g of cement
is mixed with 25 per cent water. The paste is filled in the mould of Vicat's
apparatus (Fig. 29) and the surface of the filled paste is
smoothened and levelled. A square
needle 10 mm x 10 mm attached to
the plunger is then lowered gently over the cement paste surface and is
released quickly. The plunger pierces the cement paste. The reading on the
attached scale is recorded. When the reading is 5-7 mm from the bottom of the
mould, the amount of water added is considered to be the correct percentage of
water for normal consistency.
Determination of Initial and Final Setting Times
When water is added to cement,
the resulting paste starts to stiffen and gain strength and lose the
consistency simultaneously. The term setting implies solidification of the
plastic cement paste. Initial and final setting times may be regarded as the
two stiffening states of the cement. The beginning of solidification, called
the initial set, marks the point in time when the paste has become unworkable.
The time taken to solidify completely marks the final set, which should not be
too long in order to resume construction activity within a reasonable time
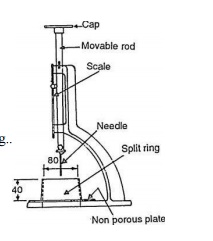
after the placement of concrete. Vicat's apparatus used for the purpose is
shown in Fig.. The initial setting time may be defined as the time taken by the
paste to stiffen to such an extent that the Vicat's needle is not permitted to
move down through the paste to within 5 ± 0.5 mm measured from the bottom of
the mould. The final setting time is the time after which the paste becomes so
hard that the angular attachment to the needle, under standard weight, fails to
leave any mark on the hardened concrete. Initial and final setting times are
the rheological properties of cement.
Importance: It is important to
know the initial setting time, because of loss of useful properties of cement
if the cement mortar or concrete is placed in moulds after this time. The
importance of final setting time lies in the fact that the moulds can be
removed after this time. The former defines the limit of handling and the
latter defines the beginning of development of machanical strength.
Conditions Affecting Setting
Time: The factors influencing the setting properties of cement are its
composition, the percentage of retardant, degree of calcination, fineness of
grinding, aeration subsequent to grinding clinker, percentage of water used to
make cement paste, the temperature of the mixing water, cement and the
atmosphere where the cement paste is placed, and the amount of manipulation the
paste receives.The effect of lime, silica and alumina in controlling the set have
been discussed in Sec. 5.3. The effect of gypsum is to increase the setting
time of freshly ground cement. It is usually mixed with the clinker before
final grinding, or just after the clinker has received preliminary grinding.
The addition of gypsum before calcination causes it to decompose into lime and
sulphur trioxide. Since the latter is liberated in the kiln, there is resulting
effect on the setting time. Often, an underlimed cement becomes quick setting
after seasoning. This can be avoided by adding to the cement 1 or 2 per cent of
hydrated lime or the fraction of a per cent of Plaster of Paris. Setting time
of cement is rapid with the increase in the fineness of cement. When the mixing
water used in testing cement paste is increased by 1 per cent above that
required for normal consistency, an increase of about 30 minutes or more is
observed in the initial or final set.
Cements stored in warm rooms
will, in general, be quick setting than those stored in cold places. Cold
mixing water retards set while warm water accelerates it. Cement exposed to
thoroughly saturated atmosphere will set much more slowly than those exposed to
a dry atmosphere. If, however, a considerable proportion of moist CO2
is present in the air, the setting time is found to reduce greatly. By
lengthening the time of mixing and by prolonged troweling of the surface
mortars, it is also possible to considerably delay the setting time.
Test Procedure: A neat cement
paste is prepared by gauging cement with 0.85 times the water required to give
a paste of standard consistency. The stop watch is started at the instant water
is added to the cement. The mould resting on a nonporous plate is filled
completely with cement
paste and the surface of filled
paste is levelled smooth with the top of the mould. The test is conducted
at room temperature of 27± 2 o C. The mould with the cement paste is placed in
the Vicat's
apparatus as shown in Fig. 5.9
and the needle is lowered gently in contact with the test block and is then
quickly released. The needle thus penetrates the test block and the reading on
the Vicat's
apparatus graduated scale is
recorded. The procedure is repeated until the needle fails to pierce the block
by about 5 mm measured from the bottom of the mould. The stop watch is pushed off
and the time is recorded which gives the initial setting time.
The
cement is considered to be finally set when upon applying the needle gently to
the surface of test block, the needle makes an impression, but the attachment
fails to do so.
Soundness Test
It is essential that the cement
concrete does not undergo large change in volume after setting. This is ensured
by limiting the quantities of free lime and magnesia which slake slowly causing
change in volume of cement (known as unsound). Soundness of cement may be
tested by Le-Chatelier method or by autoclave method. For OPC, RHC, LHC and PPC
it is limited to 10 mm, whereas for HAC and SSC it should not exceed 5 mm.
Importance: It is a very
important test to assure the quality of cement since an unsound cement produces
cracks, distortion and disintegration, ultimately leading to failure.
Conditions Affecting Soundness:
The main cause for unsoundness in Portland cement is the hydration of the
uncombined lime encased within the cement particles. Exposed, finely ground,
free lime in small percentages, hydrates before the cement sets and produces no
injurious effect. The uncombined lime in cement is a result of either
underburning the clinker or of excess lime in the raw materials. Freshly ground
cement is often unsound due to the presence of uncombined lime. Cement is thus
allowed to aerate for two to three weeks, allowing the lime to hydrate, to
overcome unsoundness.
Fine grinding of the raw material
and clinker help to produce a sound cement. By grinding fine the raw materials,
it is possible to produce a homogeneous mixture before burning where the lime
is uniformly distributed. The coarse grains of cement may imprison minute
particles of uncombined lime which do not hydrate. These lime particles on
hydralion produce disintegration.
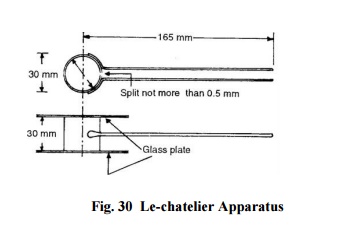
Le-chatelier
Method: The apparatus is shown in Fig. 30. The mould is placed on a
glass sheet and is filled with neat cement paste formed by gauging 100 g
cement with 0.78 times the water required to give a paste of standard
consistency. The mould is covered with a glass sheet and a small weight is
placed on the covering glass sheet. The mould is then submerged in the water at
temperature of 27 o -32 o C. After 24 hours, the mould is taken out and the
distance separating the indicator points is measured. The mould is again
submerged in water. The water is now boiled for 3 hours. The mould is removed
from water and is cooled down. The distance between the indicator points is
measured again. The difference between the two measurements represents the
unsoundness of cement.
Autoclave Test: The 25 ×
25 × 250 mm specimen is made with neat cement paste. After 24 hours the moulded
specimen is removed from the moist atmosphere, measured for length, and so
placed in an autoclave at room temperature that the four tides of each specimen
are at least exposed to saturated steam. The temperature of the autoclave is
raised at such a rate that the gauge pressure of the steam rises to 2.1 N/mm2
in 1 to 1 ¼ hours from the time the heat is turned on. The pressure is
maintained for 3 hours. Then the heat supply is shut off and the autoclave is
cooled at such a rate that the pressure is less than 0.1N/mm2 at the
end of the hour. The autoclave is then opened and the test specimens are placed
in water at temperature of 90 o C.The temperature is gradually brought down to
27±2 o C in 15 minutes. The specimens are maintained at this temperature for next
15 minutes and are then taken out. The length of the specimen is measured
again. The difference in the two measurements gives the unsoundness of the
cement.
Determination of Strength
Cement hydrates when water is
added to it and cohesion and solidity is exhibited. It binds together the
aggregates by adhesion. The strength of mortar and concrete depends upon the
type and nature of cement. So, it should develop a minimum specified strength if
it is to be used in structures. Cement is tested for compressive and tensile
strengths.
Conditions Affecting Strength:
Cement is very strong at early ages if a high lime or high alumina content is
there. Gypsum and Plaster of Paris in small percentages also tend to increase
the strength slightly, but when present in quantities larger then 3 per cent,
these substances provide variable effects. The effect of the clinker compounds
on strength have already been discussed. In addition to the effect of composition,
the strength of cement is greatly influenced by the degree of burning, the
fineness of grinding, and the aeration it receives subsequent to final
grinding. An underburnt cement is likely to be deficient in strength.
Compressive Strength: Compressive
strength is the basic data required for mix design. By this test, the quality
and the quantity of concrete can be cotrolled and the degree of adulteration
can be checked.
The test specimens are 70.6 mm
cubes having face area of about 5000 sq. mm. Large size specimen cubes cannot
be made since cement shrinks and cracks may develop. The temperature of water
and test room should be 27 o ± 2 o C. A mixture of cement and standard sand in the
proportion 1:3 by weight is mixed dry with a trowel for one minute and then
with water until the mixture is of uniform colour. Three specimen cubes are
prepared. The material for each cube is mixed separately. The quantities of
cement, standard sand and water are 185 g, 555 g and (P/4) + 3.5, respectively
where P = percentage of water required to produce a paste of standard
consistency. The mould is filled completely with the cement paste and is placed
on the vibration table. Vibrations are imparted for about 2 minutes at a speed
of 12000±4 00 per minute.
The cubes are then removed from
the moulds and submerged in clean fresh water and are taken out just prior to
testing in a compression testing machine. Compressive strength is taken to be
the average of the results of the three cubes. The load is applied starting
from zero at a rate of 35 N/sq mm/minute. The compressive strength is
calculated from the crushing load divided by the average area over which the
load is applied.The result is expressed in N/mm2. The minimum
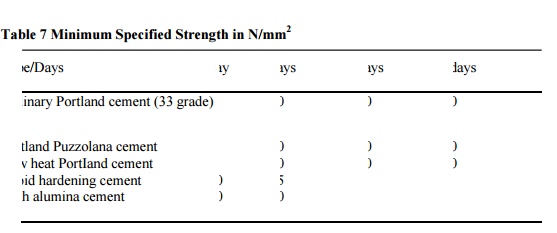
specified strength for some of the cements is given in Table 5.4.
Table 7
Minimum Specified Strength in N/mm2
Tensile Strength: The
tensile strength may be determined by Briquette test method or by split tensile
strength test.
Importance: The
tensile strength of cement affords quicker indications of defects in the cement
than any other test. Also, the test is more conveniently made than the
compressive strength test. Moreover, since the flexural strength, is directly
related to the tensile strength this test is ideally fitted to give information
both with regard to tensile and compressive strengths when the supply for
material testing is small.
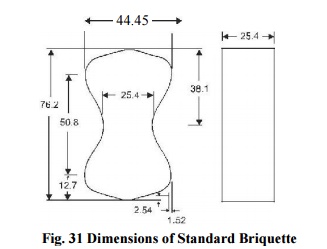
Briquette Method: A mixture
of cement and sand is gauged in the proportion of 1:3 by weight. The percentage
of water to be used is calculated from the formula (P/5) + 2.5, where P =
percentage of water required to produce a paste of standard consistency. The
temperature of the water and the test room should be 27 o ± 2 o C. The mix is
filled in the moulds of the shape shown in Fig. 5.11.
After
filling the mould, an additional heap of mix is placed on the mould and is
pushed down with the standard spatula, until the mixture is level with the top
of the mould. This operation is repeated on the other side of the mould also.
The briquettes in the mould are finished by smoothing the surface with the
blade of a trowel. They are then kept for 24 hours at a temperature of 27 o ±
2 o C and in an atmosphere having 90 per cent humidity. The briquettes are then
kept in clean fresh water and are taken out before testing. Six briquettes are
tested and the average tensile strength is calculated. Load is applied steadily
and uniformly, starting from zero and increasing at the rate of 0.7 N/sq mm of
section in 12 seconds.
Ordinary Portland cement should
have a tensile strength of not less than 2.0 N/mm2 after 3 days and
not less than 2.5 N/mm2 after 7 days.
Notes: (i) In the tension test of cement the load
on the briquette should be applied centrally. Since briquettes become brittle
with age, the effect of slight eccentricity or any torsional strain is
pronounced in long-time tests.
(ii) The strength increases when the loading rate
is increased from that specified.
Heat of hydration
Heat is evolved during hydration
of cement, the amount being dependent on the relative quantities of the clinker
compounds.
Importance: The evolution of heat
causes an increase in temperature of the concrete, being greatest in mass concreting.
Since the cooling of a mass of concrete can only occur from surfaces exposed to
atmosphere the temperature of the interior is higher than that at the surface
and also there is a rapid increase in strength in the interior than at the
surface. Shrinkage cracks may result from stresses, induced by cooling of the
surface while the interior of concrete is still at higher temperature. However,
in practice, the heat evolution may be taken to its advantage in cold weather
provided the concrete is warm at the time of placing and excessive heat loss is
prevented by suitable lagging.
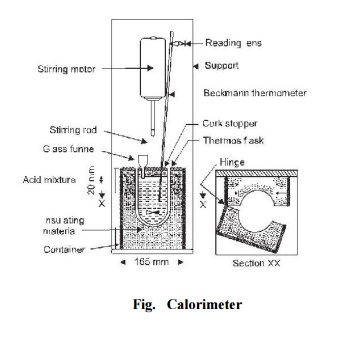
Test Procedure: The apparatus
used to determine the heat of hydration of cement is known as calorimeter and
is shown in Fig. 32. 60 g of cement and 24 ml of distilled water are mixed for
4 minutes at temperature 15 o -25 o C. Three specimen glass
vials 100 × 20 mm are filled with this mixture, corked and sealed with wax. The
vials are then stored with the mixture in a vertical position at 27 o ±2 o C. The
heat of hydration is obtained by subtracting the respective heat of solution of
unhyrated cement calculated nearest to 0.1 calorie.
For
determining the heat of solution of unhydrated cement, weigh a sample of about
3 g. At the same time, weigh out 7.0 g of cement for the loss on ignition.
Heat of solution (Cal/g) of unhydrated cement
where 0.2 is the specific heat of unhydrated cement.
For determining heat of solution
of the hydrated cement, one of the glass vials is opened and the adherent wax
is removed. The cement is ground rapidly, to avoid carbonation, to pass an 850
micron sieve. From this weigh out 4.2 g and 7.0 g of cement samples for heat of
solution and loss on ignition.
The heat
of solution of hydrated cement (Cal/g ignited weight)
Heat capacity
× corrected temperature
/ Weight of sample corrected for ignition M 0 M)
The ignition loss can be obtained
by placing the sample in a cool furnace and raising the temperature of the
furnace to 900 o C over a period of 1 hour. The sample is kept at 900 o ± 50 o C for
3 -4 hours and then cooled in a desiccator containing anhydrous calcium
chloride. Weigh after half an hour. The difference in the two weighings give
the loss on ignition.
To
determine the heat capacity sufficient quantity of zinc oxide is ignited for
one hour at 900 o ± 50 o C. It is cooled in a desiccator containing anhydrous
calcium chloride and ground to pass 250 micron sieve. About 7 g of this ignited
oxide is reheated to 900 o ± 50 o C for 5 minutes and then cooled for about 2½
hours (not more than 5 hours). The calorimeter is assembled and temperature
reading correct to 0.001 o C is recorded to determine the initial heating or
cooling correction. The zinc oxide is then introduced. The temperature readings
are recorded at one minute intervals until the solution is complete. The
recording of readings is continued for next 5 minutes to determine the final
heating or cooling correction. The initial and final heating or cooling rates
against the corresponding calorimeter temperature are plotted. The two points
thus obtained are joined by a straight line. From this graph the corrections
are read off for each temperature reading during the solution period. Heat
capacity is calculated from the expression.
Heat capcity (Cal/ o C)
= Weight of ZnO / Corrected temperature rise [256.10.1(30.0 \ 0 ) 0.1(\ 0
= Weight of ZnO (259.1 0.2\ 0.1\0 ) / Corrected
temperature rise
where, 256.1 is the heat of solution
of zinc oxide at 30 o C and 0.2 the negative temperature coefficient of the heat
of solution, is the final temperature of the calorimeter, 0.1 is the specific
heat of zinc oxide and is the room temperature in o C.
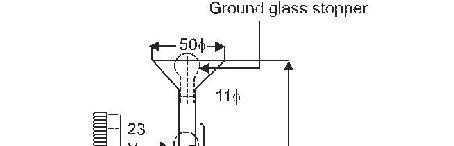
Specific Gravity Test
The
specific gravity of hydraulic cement is obtained using Le-Chatelier flask shown
in Fig. 5.13.
Conditions Affecting Specific
Gravity: Long seasonig is the chief cause of a low specific gravity in
unadulterated cement. This is because the freshly ground cement when exposed to
air rapidly absorbs moisture and carbon dioxide. Cements with high contents of
iron oxide have a higher specific gravity. The effect of fineness of grinding
upon specific gravity is slight. Very finely ground cements are likely to have
lower specific gravities.
Test Procedure: The flask is
filled with either kerosene free of water, or naphtha having a specific gravity
not less than 0.7313 to a point on the stem between zero and 1-ml mark. The
flask is immersed in a constant temperature water bath and the reading is
recorded. A weighed quantity of cement (about 64 g of Portland cement) is then
introduced in small amounts at the same temperature as that of the liquid.
After introducing all the cement, the stopper is placed in the flask and the
flask rolled in an inclined position, or gently whirled in a horizontal circle,
so as to free the cement from air until no further air bubbles rise to the
surface of the liquid. The flask is again immersed in the water-bath and the
final reading is recorded. The difference between the first and the final
reading represents the volume of liquid displaced by the weight of the cement
used in the test.